- Androgen receptor
-
Androgen_recep 
crystal structure of the human androgen receptor ligand binding domain bound with an androgen receptor nh2-terminal peptide, ar20-30, and r1881 Identifiers Symbol Androgen_recep Pfam PF02166 InterPro IPR001103 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary 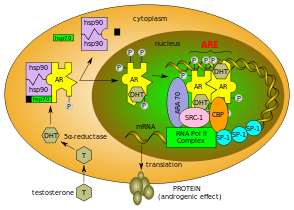 Normal function of the androgen receptor. Testosterone (T) enters the cell and, if 5-alpha-reductase is present, is converted into dihydrotestone (DHT). Upon steroid binding, the androgen receptor (AR) undergoes a conformational change and releases heat-shock proteins (hsps). Phosphorylation (P) occurs before or after steroid binding. The AR translocates to the nucleus where dimerization, DNA binding, and the recruitment of coactivators occur. Target genes are transcribed (mRNA) and translated into proteins.[2][3][4][5]
Normal function of the androgen receptor. Testosterone (T) enters the cell and, if 5-alpha-reductase is present, is converted into dihydrotestone (DHT). Upon steroid binding, the androgen receptor (AR) undergoes a conformational change and releases heat-shock proteins (hsps). Phosphorylation (P) occurs before or after steroid binding. The AR translocates to the nucleus where dimerization, DNA binding, and the recruitment of coactivators occur. Target genes are transcribed (mRNA) and translated into proteins.[2][3][4][5]
The androgen receptor (AR), also known as NR3C4 (nuclear receptor subfamily 3, group C, member 4), is a type of nuclear receptor[6] that is activated by binding of either of the androgenic hormones testosterone or dihydrotestosterone [7] in the cytoplasm and then translocating into the nucleus. The androgen receptor is most closely related to the progesterone receptor, and progestins in higher dosages can block the androgen receptor.[8][9]
The main function of the androgen receptor is as a DNA-binding transcription factor that regulates gene expression;[10] however, the androgen receptor has other functions as well.[11] Androgen regulated genes are critical for the development and maintenance of the male sexual phenotype.
Contents
Function
Effect on development
In some cell types, testosterone interacts directly with androgen receptors, whereas, in others, testosterone is converted by 5-alpha-reductase to dihydrotestosterone, an even more potent agonist for androgen receptor activation.[12] Testosterone appears to be the primary androgen receptor-activating hormone in the Wolffian duct, whereas dihydrotestosterone is the main androgenic hormone in the urogenital sinus, urogenital tubercle, and hair follicles.[13] Hence, testosterone is responsible primarily for the development of male primary sexual characteristics, whereas dihydrotestosterone is responsible for secondary male characteristics.
Androgens cause slow epiphysis, or maturation of the bones, but more of the potent epiphysis effect comes from the estrogen produced by aromatization of androgens. Steroid users of teen age may find that their growth had been stunted by androgen and/or estrogen excess. People with too little sex hormones can be short during puberty but end up taller as adults as in androgen insensitivity syndrome or estrogen insensitivity syndrome.[14]
Also, AR knockout-mice studies have shown that AR is essential for normal female fertility, being required for development and full functionality of the ovarian follicles and ovulation, working through both intra-ovarian and neuroendocrine mechanisms.[15]
Mechanism of action
Genomic
The primary mechanism of action for androgen receptors is direct regulation of gene transcription. The binding of an androgen to the androgen receptor results in a conformational change in the receptor that, in turn, causes dissociation of heat shock proteins, transport from the cytosol into the cell nucleus, and dimerization. The androgen receptor dimer binds to a specific sequence of DNA known as a hormone response element. Androgen receptors interact with other proteins in the nucleus, resulting in up- or down-regulation of specific gene transcription.[16] Up-regulation or activation of transcription results in increased synthesis of messenger RNA, which, in turn, is translated by ribosomes to produce specific proteins. One of the known target genes of androgen receptor activation is insulin-like growth factor I (IGF-1).[17] Thus, changes in levels of specific proteins in cells is one way that androgen receptors control cell behavior.
One function of androgen receptor that is independent of direct binding to its target DNA sequence, is facilitated by recruitment via other DNA-binding proteins. One example is serum response factor, a protein that activates several genes that cause muscle growth.[18]
Non-genomic
More recently, androgen receptors have been shown to have a second mode of action. As has been also found for other steroid hormone receptors such as estrogen receptors, androgen receptors can have actions that are independent of their interactions with DNA.[11][19] Androgen receptors interact with certain signal transduction proteins in the cytoplasm. Androgen binding to cytoplasmic androgen receptors can cause rapid changes in cell function independent of changes in gene transcription, such as changes in ion transport. Regulation of signal transduction pathways by cytoplasmic androgen receptors can indirectly lead to changes in gene transcription, for example, by leading to phosphorylation of other transcription factors.
Genetics
Gene
In humans, the androgen receptor is encoded by the AR gene located on the X chromosome at Xq11-12.[20][21]
AR deficiencies
The androgen insensitivity syndrome, formerly known as testicular feminization, is caused by a mutation of the Androgen Receptor gene located on the X chromosome (locus:Xq11-Xq12).[22] The androgen receptor seems to affect neuron physiology and is defective in Kennedy disease.[23][24] In addition, point mutations and trinucleotide repeat polymorphisms has been linked to a number of additional disorders.[25]
Structure
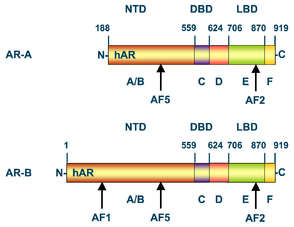 Structural domains of the two isoforms (AR-A and AR-B) of the human androgen receptor. Numbers above the bars refer to the amino acid residues that separate the domains starting from the N-terminus (left) to C-terminus (right). NTD = N-terminal domain, DBD = DNA binding domain. LBD = ligand binding domain. AF = activation function.
Structural domains of the two isoforms (AR-A and AR-B) of the human androgen receptor. Numbers above the bars refer to the amino acid residues that separate the domains starting from the N-terminus (left) to C-terminus (right). NTD = N-terminal domain, DBD = DNA binding domain. LBD = ligand binding domain. AF = activation function.
Isoforms
Two isoforms of the androgen receptor (A and B) have been identified:[26]
- AR-A - 87 kDa - N-terminus truncated (lacks the first 187 amino acids), which results from in vitro proteolysis.[27]
- AR-B - 110 kDa - full length
Domains
Like other nuclear receptors, the androgen receptor is modular in structure and is composed of the following functional domains labeled A through F:[28]
- A/B) - N-terminal regulatory domain contains:[29]
- activation function 1 (AF-1) between residues 101 and 370 required for full ligand activated transcriptional activity
- activation function 5 (AF-5) between residues 360-485 is responsible for the constitutive activity (activity without bound ligand)
- dimerization surface involving residues 1-36 (containing the FXXLF motif where F = phenylalanine, L = leucine, and X = any amino acid residue) and 370-494, both of which interact with the LBD in an intramolecular[30][31][32] head-to-tail interaction[33][34][35]
- C) - DNA binding domain (DBD)
- D) - Hinge region - flexible region that connects the DBD with the LBD; along with the DBD, contains a ligand dependent nuclear localization signal[36]
- E) - Ligand binding domain (LBD) containing
- activation function 2 (AF-2), responsible for agonist induced activity (activity in the presence of bound agonist)
- AF-2 binds either the N-terminal FXXFL motif intramolecularly or coactivator proteins (containing the LXXLL or preferably FXXFL motifs)[35]
- A ligand dependent nuclear export signal[37]
- F) - C-terminal domain
Interactions
Androgen receptor has been shown to interact with:
- AKT1,[38]
- BAG1,[39][40][41]
- Beta-catenin,[42][43][44][45][46][47]
- BRCA1,[48][49]
- C-jun,[50]
- Calmodulin 1,[51]
- Caveolin 1,[52]
- CDK9,[53]
- COX5B,[54]
- CREB-binding protein,[55][56][57][58]
- Cyclin D1,[59][60]
- Cyclin-dependent kinase 7,[61]
- Death associated protein 6,[62]
- Deleted in Colorectal Cancer,[63]
- EFCAB6,[64]
- Epidermal growth factor receptor,[65][66]
- FOXO1,[67]
- GAPDH,[68]
- Gelsolin,[69]
- GNB2L1,[70]
- GSK3B,[71]
- HDAC1,[72]
- HSP90AA1,[73][74]
- HTATIP,[72]
- MAGEA11,[75][76]
- MED1,[77]
- MYST2,[78]
- NCOA1,[43][79][80]
- NCOA2,[42][57][75][81][82]
- NCOA3,[81][83][84]
- NCOA4,[38][82][85][86][87][88][89][90][91]
- NCOA6,[92]
- NCOR2,[42][93][94]
- NONO,[57]
- PA2G4,[95]
- PAK6,[96][97]
- PATZ1,[98]
- PIAS2,[99][100]
- PRPF6,[101]
- PTEN,[102]
- RAD9A,[103]
- RANBP9,[104]
- RCHY1,[105]
- Retinoblastoma protein,[106][107]
- RNF14,[82][85][108][109]
- RNF4,[98][110][111]
- SART3,[112]
- SMAD3,[113][114][115]
- Small heterodimer partner,[116]
- Src,[102][117][118]
- SRY,[119]
- STAT3,[120][121]
- SVIL,[122]
- Testicular receptor 2,[123]
- Testicular receptor 4,[124]
- TGFB1I1,[85][125]
- TMF1,[126]
- TRIM68,[127]
- UBE2I,[42][43][128][129][130][131]
- UXT,[132] and
- ZMIZ1.[133]
See also
References
- ^ PDB 2AM9; Pereira de Jésus-Tran K, Côté PL, Cantin L, Blanchet J, Labrie F, Breton R (May 2006). "Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity". Protein Sci. 15 (5): 987–99. doi:10.1110/ps.051905906. PMC 2242507. PMID 16641486. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2242507.
- ^ Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS (June 1995). "Androgen receptor defects: historical, clinical, and molecular perspectives". Endocr. Rev. 16 (3): 271–321. PMID 7671849.
- ^ Gottlieb B, Lombroso R, Beitel LK, Trifiro MA (January 2005). "Molecular pathology of the androgen receptor in male (in)fertility". Reprod. Biomed. Online 10 (1): 42–8. doi:10.1016/S1472-6483(10)60802-4. PMID 15705293.
- ^ Choong CS, Wilson EM (December 1998). "Trinucleotide repeats in the human androgen receptor: a molecular basis for disease". J. Mol. Endocrinol. 21 (3): 235–57. doi:10.1677/jme.0.0210235. PMID 9845666.
- ^ Meehan KL, Sadar MD (May 2003). "Androgens and androgen receptor in prostate and ovarian malignancies". Front. Biosci. 8: d780–800. doi:10.2741/1063. PMID 12700055.
- ^ Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA (December 2006). "International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors". Pharmacol. Rev. 58 (4): 782–97. doi:10.1124/pr.58.4.9. PMID 17132855.
- ^ Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B (1999). "Regulation of androgen action". Vitam. Horm.. Vitamins & Hormones 55: 309–52. doi:10.1016/S0083-6729(08)60938-3. ISBN 9780127098555. PMID 9949684.
- ^ Bardin CW, Brown T, Isomaa VV, Jänne OA (1983). "Progestins can mimic, inhibit and potentiate the actions of androgens". Pharmacol. Ther. 23 (3): 443–59. doi:10.1016/0163-7258(83)90023-2. PMID 6371845.
- ^ Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226. http://drugs.adisonline.com/pt/re/drugs/abstract.00003495-200363050-00003.htm.
- ^ Mooradian AD, Morley JE, Korenman SG (1987). "Biological actions of androgens". Endocr. Rev. 8 (1): 1–28. doi:10.1210/edrv-8-1-1. PMID 3549275.
- ^ a b Heinlein CA, Chang C (2002). "The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions". Mol. Endocrinol. 16 (10): 2181–7. doi:10.1210/me.2002-0070. PMID 12351684.
- ^ Davison SL, Bell R (April 2006). "Androgen physiology". Semin. Reprod. Med. 24 (2): 71–7. doi:10.1055/s-2006-939565. PMID 16633980.
- ^ Sinisi AA, Pasquali D, Notaro A, Bellastella A (2003). "Sexual differentiation". J. Endocrinol. Invest. 26 (3 Suppl): 23–8. PMID 12834017.
- ^ Frank GR (September 2003). "Role of estrogen and androgen in pubertal skeletal physiology". Med. Pediatr. Oncol. 41 (3): 217–21. doi:10.1002/mpo.10340. PMID 12868122.
- ^ Walters KA, Simanainen U, Handelsman DJ (March 2010). "Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models". Hum Reprod Update 16 (5): 543–58. doi:10.1093/humupd/dmq003. PMID 20231167.
- ^ Heemers HV, Tindall DJ (December 2007). "Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex". Endocr. Rev. 28 (7): 778–808. doi:10.1210/er.2007-0019. PMID 17940184.
- ^ Pandini G, Mineo R, Frasca F, Roberts CT Jr, Marcelli M, Vigneri R, Belfiore A (March 2005). "Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells". Cancer Res. 65 (5): 1849–57. doi:10.1158/0008-5472.CAN-04-1837. PMID 15753383.
- ^ Vlahopoulos S, Zimmer WE, Jenster G, Belaguli NS, Balk SP, Brinkmann AO, Lanz RB, Zoumpourlis VC, Schwartz RJ (2005). "Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene". J. Biol. Chem. 280 (9): 7786–92. doi:10.1074/jbc.M413992200. PMID 15623502.
- ^ Fix C, Jordan C, Cano P, Walker WH (2004). "Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells". Proc Natl Acad Sci USA 101 (30): 10919–24. doi:10.1073/pnas.0404278101. PMC 503720. PMID 15263086. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=503720.
- ^ Chang CS, Kokontis J, Liao ST (1988). "Molecular cloning of human and rat complementary DNA encoding androgen receptors". Science 240 (4850): 324–6. doi:10.1126/science.3353726. PMID 3353726.
- ^ Trapman J, Klaassen P, Kuiper GG, van der Korput JA, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Brinkmann AO (1988). "Cloning, structure and expression of a cDNA encoding the human androgen receptor". Biochem. Biophys. Res. Commun. 153 (1): 241–8. doi:10.1016/S0006-291X(88)81214-2. PMID 3377788.
- ^ Brown TR (1995). "Human androgen insensitivity syndrome" (abstract). J. Androl. 16 (4): 299–303. PMID 8537246. http://www.andrologyjournal.org/cgi/content/abstract/16/4/299.
- ^ Kennedy WR, Alter M, Sung JH (1968). "Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait". Neurology 18 (7): 671–80. PMID 4233749.
- ^ Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP (2006). "Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model". J. Clin. Invest. 116 (10): 2663–72. doi:10.1172/JCI28773. PMC 1564432. PMID 16981011. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1564432.
- ^ Rajender S, Singh L, Thangaraj K (2007). "Phenotypic heterogeneity of mutations in androgen receptor gene". Asian J. Androl. 9 (2): 147–79. doi:10.1111/j.1745-7262.2007.00250.x. PMID 17334586.
- ^ Wilson CM, McPhaul MJ (1994). "A and B forms of the androgen receptor are present in human genital skin fibroblasts". Proc. Natl. Acad. Sci. U.S.A. 91 (4): 1234–8. doi:10.1073/pnas.91.4.1234. PMC 43131. PMID 8108393. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=43131.
- ^ Gregory CW, He B, Wilson EM. (2001). "The putative androgen receptor-A form results from in vitro proteolysis". J Mol Endocrinol 27 (3): 309–19. doi:10.1677/jme.0.0270309. PMID 11719283.
- ^ Brinkmann AO, Klaasen P, Kuiper GG, van der Korput JA, Bolt J, de Boer W, Smit A, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Trapman J (1989). "Structure and function of the androgen receptor". Urol. Res. 17 (2): 87–93. doi:10.1007/BF00262026. PMID 2734982.
- ^ Jenster G, van der Korput HA, Trapman J, Brinkmann AO (1995). "Identification of two transcription activation units in the N-terminal domain of the human androgen receptor". J. Biol. Chem. 270 (13): 7341–6. doi:10.1074/jbc.270.13.7341. PMID 7706276.
- ^ Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, Miner JN, Diamond MI (July 2005). "The structural basis of androgen receptor activation: Intramolecular and intermolecular amino–carboxy interactions". Proc. Natl. Acad. Sci. U.S.A. 102 (28): 9802–7. doi:10.1073/pnas.0408819102. PMC 1168953. PMID 15994236. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1168953.
- ^ Klokk TI, Kurys P, Elbi C, Nagaich AK, Hendarwanto A, Slagsvold T, Chang CY, Hager GL, Saatcioglu F (March 2007). "Ligand-Specific Dynamics of the Androgen Receptor at Its Response Element in Living Cells". Mol. Cell. Biol. 27 (5): 1823–43. doi:10.1128/MCB.01297-06. PMC 1820481. PMID 17189428. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1820481.
- ^ van Royen ME, Cunha SM, Brink MC, Mattern KA, Nigg AL, Dubbink HJ, Verschure PJ, Trapman J, Houtsmuller AB (April 2007). "Compartmentalization of androgen receptor protein–protein interactions in living cells". J. Cell Biol. 177 (1): 63–72. doi:10.1083/jcb.200609178. PMC 2064112. PMID 17420290. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2064112.
- ^ Langley E, Zhou ZX, Wilson EM (1995). "Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer". J. Biol. Chem. 270 (50): 29983–90. doi:10.1074/jbc.270.50.29983. PMID 8530400.
- ^ Berrevoets CA, Doesburg P, Steketee K, Trapman J, Brinkmann AO (1998). "Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor2)". Mol. Endocrinol. 12 (8): 1172–83. doi:10.1210/me.12.8.1172. PMID 9717843.
- ^ a b Dubbink HJ, Hersmus R, Verma CS, van der Korput HA, Berrevoets CA, van Tol J, Ziel-van der Made AC, Brinkmann AO, Pike AC, Trapman J (2004). "Distinct recognition modes of FXXLF and LXXLL motifs by the androgen receptor". Mol. Endocrinol. 18 (9): 2132–50. doi:10.1210/me.2003-0375. PMID 15178743.
- ^ Kaku N, Matsuda KI, Tsujimura A, Kawata M (April 2008). "Characterization of Nuclear Import of the Domain-Specific Androgen Receptor in Association with the Importin α/β and Ran-Guanosine 5′-Triphosphate Systems". Endocrinology 149 (8): 3960–9. doi:10.1210/en.2008-0137. PMC 2488236. PMID 18420738. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2488236.
- ^ Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z (October 2003). "Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor". J. Biol. Chem. 278 (43): 41998–2005. doi:10.1074/jbc.M302460200. PMID 12923188.
- ^ a b Lin HK, Yeh S, Kang HY, Chang C (June 2001). "Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor". Proc. Natl. Acad. Sci. U.S.A. 98 (13): 7200–5. doi:10.1073/pnas.121173298. PMC 34646. PMID 11404460. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=34646.
- ^ Shatkina L, Mink S, Rogatsch H, Klocker H, Langer G, Nestl A, Cato AC (October 2003). "The Cochaperone Bag-1L Enhances Androgen Receptor Action via Interaction with the NH2-Terminal Region of the Receptor". Mol. Cell. Biol. 23 (20): 7189–97. doi:10.1128/MCB.23.20.7189-7197.2003. PMC 230325. PMID 14517289. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=230325.
- ^ Knee DA, Froesch BA, Nuber U, Takayama S, Reed JC (April 2001). "Structure-function analysis of Bag1 proteins. Effects on androgen receptor transcriptional activity". J. Biol. Chem. 276 (16): 12718–24. doi:10.1074/jbc.M010841200. PMID 11278763.
- ^ Froesch BA, Takayama S, Reed JC (May 1998). "BAG-1L protein enhances androgen receptor function". J. Biol. Chem. 273 (19): 11660–6. doi:10.1074/jbc.273.19.11660. PMID 9565586.
- ^ a b c d Song LN, Coghlan M, Gelmann EP (January 2004). "Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor". Mol. Endocrinol. 18 (1): 70–85. doi:10.1210/me.2003-0189. PMID 14593076.
- ^ a b c Masiello D, Chen SY, Xu Y, Verhoeven MC, Choi E, Hollenberg AN, Balk SP (October 2004). "Recruitment of beta-catenin by wild-type or mutant androgen receptors correlates with ligand-stimulated growth of prostate cancer cells". Mol. Endocrinol. 18 (10): 2388–401. doi:10.1210/me.2003-0436. PMID 15256534.
- ^ Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z (March 2002). "Linking beta-catenin to androgen-signaling pathway". J. Biol. Chem. 277 (13): 11336–44. doi:10.1074/jbc.M111962200. PMID 11792709.
- ^ Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP (August 2003). "A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4". J. Biol. Chem. 278 (33): 30828–34. doi:10.1074/jbc.M301208200. PMID 12799378.
- ^ Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC (August 2003). "Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis". Oncogene 22 (36): 5602–13. doi:10.1038/sj.onc.1206802. PMID 12944908.
- ^ Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME (June 2002). "Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells". J. Biol. Chem. 277 (23): 20702–10. doi:10.1074/jbc.M200545200. PMID 11916967.
- ^ Park JJ, Irvine RA, Buchanan G, Koh SS, Park JM, Tilley WD, Stallcup MR, Press MF, Coetzee GA (November 2000). "Breast cancer susceptibility gene 1 (BRCAI) is a coactivator of the androgen receptor". Cancer Res. 60 (21): 5946–9. PMID 11085509.
- ^ Yeh S, Hu YC, Rahman M, Lin HK, Hsu CL, Ting HJ, Kang HY, Chang C (October 2000). "Increase of androgen-induced cell death and androgen receptor transactivation by BRCA1 in prostate cancer cells". Proc. Natl. Acad. Sci. U.S.A. 97 (21): 11256–61. doi:10.1073/pnas.190353897. PMC 17187. PMID 11016951. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17187.
- ^ Sato N, Sadar MD, Bruchovsky N, Saatcioglu F, Rennie PS, Sato S, Lange PH, Gleave ME (July 1997). "Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP". J. Biol. Chem. 272 (28): 17485–94. doi:10.1074/jbc.272.28.17485. PMID 9211894.
- ^ Cifuentes E, Mataraza JM, Yoshida BA, Menon M, Sacks DB, Barrack ER, Reddy GP (January 2004). "Physical and functional interaction of androgen receptor with calmodulin in prostate cancer cells". Proc. Natl. Acad. Sci. U.S.A. 101 (2): 464–9. doi:10.1073/pnas.0307161101. PMC 327170. PMID 14695896. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=327170.
- ^ Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP (April 2001). "Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation". J. Biol. Chem. 276 (16): 13442–51. doi:10.1074/jbc.M006598200. PMID 11278309.
- ^ Lee DK, Duan HO, Chang C (March 2001). "Androgen receptor interacts with the positive elongation factor P-TEFb and enhances the efficiency of transcriptional elongation". J. Biol. Chem. 276 (13): 9978–84. doi:10.1074/jbc.M002285200. PMID 11266437.
- ^ Beauchemin AM, Gottlieb B, Beitel LK, Elhaji YA, Pinsky L, Trifiro MA (2001). "Cytochrome c oxidase subunit Vb interacts with human androgen receptor: a potential mechanism for neurotoxicity in spinobulbar muscular atrophy". Brain Res. Bull. 56 (3–4): 285–97. doi:10.1016/S0361-9230(01)00583-4. PMID 11719263.
- ^ Kim J, Jia L, Stallcup MR, Coetzee GA (February 2005). "The role of protein kinase A pathway and cAMP responsive element-binding protein in androgen receptor-mediated transcription at the prostate-specific antigen locus". J. Mol. Endocrinol. 34 (1): 107–18. doi:10.1677/jme.1.01701. PMID 15691881.
- ^ Frønsdal K, Engedal N, Slagsvold T, Saatcioglu F (November 1998). "CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1". J. Biol. Chem. 273 (48): 31853–9. doi:10.1074/jbc.273.48.31853. PMID 9822653.
- ^ a b c Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S (July 2003). "p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor". Biochem. Biophys. Res. Commun. 306 (3): 660–5. doi:10.1016/S0006-291X(03)01021-0. PMID 12810069.
- ^ Aarnisalo P, Palvimo JJ, Jänne OA (March 1998). "CREB-binding protein in androgen receptor-mediated signaling". Proc. Natl. Acad. Sci. U.S.A. 95 (5): 2122–7. doi:10.1073/pnas.95.5.2122. PMC 19270. PMID 9482849. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=19270.
- ^ Petre-Draviam CE, Williams EB, Burd CJ, Gladden A, Moghadam H, Meller J, Diehl JA, Knudsen KE (January 2005). "A central domain of cyclin D1 mediates nuclear receptor corepressor activity". Oncogene 24 (3): 431–44. doi:10.1038/sj.onc.1208200. PMID 15558026.
- ^ Knudsen KE, Cavenee WK, Arden KC (May 1999). "D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability". Cancer Res. 59 (10): 2297–301. PMID 10344732.
- ^ Lee DK, Duan HO, Chang C (March 2000). "From androgen receptor to the general transcription factor TFIIH. Identification of cdk activating kinase (CAK) as an androgen receptor NH(2)-terminal associated coactivator". J. Biol. Chem. 275 (13): 9308–13. doi:10.1074/jbc.275.13.9308. PMID 10734072.
- ^ Lin DY, Fang HI, Ma AH, Huang YS, Pu YS, Jenster G, Kung HJ, Shih HM (December 2004). "Negative Modulation of Androgen Receptor Transcriptional Activity by Daxx". Mol. Cell. Biol. 24 (24): 10529–41. doi:10.1128/MCB.24.24.10529-10541.2004. PMC 533990. PMID 15572661. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=533990.
- ^ Wafa LA, Cheng H, Rao MA, Nelson CC, Cox M, Hirst M, Sadowski I, Rennie PS (October 2003). "Isolation and identification of L-dopa decarboxylase as a protein that binds to and enhances transcriptional activity of the androgen receptor using the repressed transactivator yeast two-hybrid system". Biochem. J. 375 (Pt 2): 373–83. doi:10.1042/BJ20030689. PMC 1223690. PMID 12864730. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1223690.
- ^ Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SM, Ariga H (February 2003). "DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex". Mol. Cancer Res. 1 (4): 247–61. PMID 12612053.
- ^ Bonaccorsi L, Carloni V, Muratori M, Formigli L, Zecchi S, Forti G, Baldi E (October 2004). "EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR)". Int. J. Cancer 112 (1): 78–86. doi:10.1002/ijc.20362. PMID 15305378.
- ^ Bonaccorsi L, Muratori M, Carloni V, Marchiani S, Formigli L, Forti G, Baldi E (August 2004). "The androgen receptor associates with the epidermal growth factor receptor in androgen-sensitive prostate cancer cells". Steroids 69 (8–9): 549–52. doi:10.1016/j.steroids.2004.05.011. PMID 15288768.
- ^ Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W (January 2003). "AKT-Independent Protection of Prostate Cancer Cells from Apoptosis Mediated through Complex Formation between the Androgen Receptor and FKHR". Mol. Cell. Biol. 23 (1): 104–18. doi:10.1128/MCB.23.1.104-118.2003. PMC 140652. PMID 12482965. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140652.
- ^ Koshy B, Matilla T, Burright EN, Merry DE, Fischbeck KH, Orr HT, Zoghbi HY (September 1996). "Spinocerebellar ataxia type-1 and spinobulbar muscular atrophy gene products interact with glyceraldehyde-3-phosphate dehydrogenase". Hum. Mol. Genet. 5 (9): 1311–8. doi:10.1093/hmg/5.9.1311. PMID 8872471.
- ^ Nishimura K, Ting HJ, Harada Y, Tokizane T, Nonomura N, Kang HY, Chang HC, Yeh S, Miyamoto H, Shin M, Aozasa K, Okuyama A, Chang C (August 2003). "Modulation of androgen receptor transactivation by gelsolin: a newly identified androgen receptor coregulator". Cancer Res. 63 (16): 4888–94. PMID 12941811.
- ^ Rigas AC, Ozanne DM, Neal DE, Robson CN (November 2003). "The scaffolding protein RACK1 interacts with androgen receptor and promotes cross-talk through a protein kinase C signaling pathway". J. Biol. Chem. 278 (46): 46087–93. doi:10.1074/jbc.M306219200. PMID 12958311.
- ^ Wang L, Lin HK, Hu YC, Xie S, Yang L, Chang C (July 2004). "Suppression of androgen receptor-mediated transactivation and cell growth by the glycogen synthase kinase 3 beta in prostate cells". J. Biol. Chem. 279 (31): 32444–52. doi:10.1074/jbc.M313963200. PMID 15178691.
- ^ a b Gaughan L, Logan IR, Cook S, Neal DE, Robson CN (July 2002). "Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor". J. Biol. Chem. 277 (29): 25904–13. doi:10.1074/jbc.M203423200. PMID 11994312.
- ^ Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E (March 1992). "Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation". Biochemistry 31 (8): 2393–9. doi:10.1021/bi00123a026. PMID 1540595.
- ^ Nemoto T, Ohara-Nemoto Y, Ota M (September 1992). "Association of the 90-kDa heat shock protein does not affect the ligand-binding ability of androgen receptor". J. Steroid Biochem. Mol. Biol. 42 (8): 803–12. doi:10.1016/0960-0760(92)90088-Z. PMID 1525041.
- ^ a b Bai S, He B, Wilson EM (February 2005). "Melanoma Antigen Gene Protein MAGE-11 Regulates Androgen Receptor Function by Modulating the Interdomain Interaction". Mol. Cell. Biol. 25 (4): 1238–57. doi:10.1128/MCB.25.4.1238-1257.2005. PMC 548016. PMID 15684378. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=548016.
- ^ Bai S, Wilson EM (March 2008). "Epidermal Growth Factor-Dependent Phosphorylation and Ubiquitinylation of MAGE-11 Regulates Its Interaction with the Androgen Receptor". Mol. Cell. Biol. 28 (6): 1947–63. doi:10.1128/MCB.01672-07. PMC 2268407. PMID 18212060. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2268407.
- ^ Wang Q, Sharma D, Ren Y, Fondell JD (November 2002). "A coregulatory role for the TRAP-mediator complex in androgen receptor-mediated gene expression". J. Biol. Chem. 277 (45): 42852–8. doi:10.1074/jbc.M206061200. PMID 12218053.
- ^ Sharma M, Zarnegar M, Li X, Lim B, Sun Z (November 2000). "Androgen receptor interacts with a novel MYST protein, HBO1". J. Biol. Chem. 275 (45): 35200–8. doi:10.1074/jbc.M004838200. PMID 10930412.
- ^ Ueda T, Mawji NR, Bruchovsky N, Sadar MD (October 2002). "Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells". J. Biol. Chem. 277 (41): 38087–94. doi:10.1074/jbc.M203313200. PMID 12163482.
- ^ Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG (December 1999). "The AF1 and AF2 Domains of the Androgen Receptor Interact with Distinct Regions of SRC1". Mol. Cell. Biol. 19 (12): 8383–92. PMC 84931. PMID 10567563. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=84931.
- ^ a b Wang Q, Udayakumar TS, Vasaitis TS, Brodie AM, Fondell JD (April 2004). "Mechanistic relationship between androgen receptor polyglutamine tract truncation and androgen-dependent transcriptional hyperactivity in prostate cancer cells". J. Biol. Chem. 279 (17): 17319–28. doi:10.1074/jbc.M400970200. PMID 14966121.
- ^ a b c He B, Wilson EM (March 2003). "Electrostatic Modulation in Steroid Receptor Recruitment of LXXLL and FXXLF Motifs". Mol. Cell. Biol. 23 (6): 2135–50. doi:10.1128/MCB.23.6.2135-2150.2003. PMC 149467. PMID 12612084. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=149467.
- ^ Tan JA, Hall SH, Petrusz P, French FS (September 2000). "Thyroid receptor activator molecule, TRAM-1, is an androgen receptor coactivator". Endocrinology 141 (9): 3440–50. doi:10.1210/en.141.9.3440. PMID 10965917.
- ^ Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN (December 2001). "Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer". Br. J. Cancer 85 (12): 1928–36. doi:10.1054/bjoc.2001.2179. PMC 2364015. PMID 11747336. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2364015.
- ^ a b c He B, Minges JT, Lee LW, Wilson EM (March 2002). "The FXXLF motif mediates androgen receptor-specific interactions with coregulators". J. Biol. Chem. 277 (12): 10226–35. doi:10.1074/jbc.M111975200. PMID 11779876.
- ^ Alen P, Claessens F, Schoenmakers E, Swinnen JV, Verhoeven G, Rombauts W, Peeters B (January 1999). "Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1alpha with multiple steroid receptors and identification of an internally deleted ELE1beta isoform". Mol. Endocrinol. 13 (1): 117–28. doi:10.1210/me.13.1.117. PMID 9892017.
- ^ Yeh S, Chang C (May 1996). "Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells". Proc. Natl. Acad. Sci. U.S.A. 93 (11): 5517–21. doi:10.1073/pnas.93.11.5517. PMC 39278. PMID 8643607. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=39278.
- ^ Miyamoto H, Yeh S, Wilding G, Chang C (June 1998). "Promotion of agonist activity of antiandrogens by the androgen receptor coactivator, ARA70, in human prostate cancer DU145 cells". Proc. Natl. Acad. Sci. U.S.A. 95 (13): 7379–84. doi:10.1073/pnas.95.13.7379. PMC 22623. PMID 9636157. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=22623.
- ^ Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C (May 1999). "From HER2/Neu signal cascade to androgen receptor and its coactivators: A novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells". Proc. Natl. Acad. Sci. U.S.A. 96 (10): 5458–63. doi:10.1073/pnas.96.10.5458. PMC 21881. PMID 10318905. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=21881.
- ^ Zhou ZX, He B, Hall SH, Wilson EM, French FS (February 2002). "Domain interactions between coregulator ARA(70) and the androgen receptor (AR)". Mol. Endocrinol. 16 (2): 287–300. doi:10.1210/me.16.2.287. PMID 11818501.
- ^ Gao T, Brantley K, Bolu E, McPhaul MJ (October 1999). "RFG (ARA70, ELE1) interacts with the human androgen receptor in a ligand-dependent fashion, but functions only weakly as a coactivator in cotransfection assays". Mol. Endocrinol. 13 (10): 1645–56. doi:10.1210/me.13.10.1645. PMID 10517667.
- ^ Goo YH, Na SY, Zhang H, Xu J, Hong S, Cheong J, Lee SK, Lee JW (February 2004). "Interactions between activating signal cointegrator-2 and the tumor suppressor retinoblastoma in androgen receptor transactivation". J. Biol. Chem. 279 (8): 7131–5. doi:10.1074/jbc.M312563200. PMID 14645241.
- ^ Liao G, Chen LY, Zhang A, Godavarthy A, Xia F, Ghosh JC, Li H, Chen JD (February 2003). "Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT". J. Biol. Chem. 278 (7): 5052–61. doi:10.1074/jbc.M206374200. PMID 12441355.
- ^ Dotzlaw H, Moehren U, Mink S, Cato AC, Iñiguez Lluhí JA, Baniahmad A (April 2002). "The amino terminus of the human AR is target for corepressor action and antihormone agonism". Mol. Endocrinol. 16 (4): 661–73. doi:10.1210/me.16.4.661. PMID 11923464.
- ^ Zhang Y, Fondell JD, Wang Q, Xia X, Cheng A, Lu ML, Hamburger AW (August 2002). "Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, Ebp1". Oncogene 21 (36): 5609–18. doi:10.1038/sj.onc.1205638. PMID 12165860.
- ^ Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z (May 2001). "Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6". J. Biol. Chem. 276 (18): 15345–53. doi:10.1074/jbc.M010311200. PMID 11278661.
- ^ Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP (January 2002). [10.1210/me.16.1.85 "AR and ER interaction with a p21-activated kinase (PAK6)"]. Mol. Endocrinol. 16 (1): 85–99. doi:10.1210/me.16.1.85. PMID 11773441. 10.1210/me.16.1.85.
- ^ a b Pero R, Lembo F, Palmieri EA, Vitiello C, Fedele M, Fusco A, Bruni CB, Chiariotti L (February 2002). "PATZ attenuates the RNF4-mediated enhancement of androgen receptor-dependent transcription". J. Biol. Chem. 277 (5): 3280–5. doi:10.1074/jbc.M109491200. PMID 11719514.
- ^ Kotaja N, Aittomäki S, Silvennoinen O, Palvimo JJ, Jänne OA (December 2000). "ARIP3 (androgen receptor-interacting protein 3) and other PIAS (protein inhibitor of activated STAT) proteins differ in their ability to modulate steroid receptor-dependent transcriptional activation". Mol. Endocrinol. 14 (12): 1986–2000. doi:10.1210/me.14.12.1986. PMID 11117529.
- ^ Moilanen AM, Karvonen U, Poukka H, Yan W, Toppari J, Jänne OA, Palvimo JJ (February 1999). "A testis-specific androgen receptor coregulator that belongs to a novel family of nuclear proteins". J. Biol. Chem. 274 (6): 3700–4. doi:10.1074/jbc.274.6.3700. PMID 9920921.
- ^ Zhao Y, Goto K, Saitoh M, Yanase T, Nomura M, Okabe T, Takayanagi R, Nawata H (August 2002). "Activation function-1 domain of androgen receptor contributes to the interaction between subnuclear splicing factor compartment and nuclear receptor compartment. Identification of the p102 U5 small nuclear ribonucleoprotein particle-binding protein as a coactivator for the receptor". J. Biol. Chem. 277 (33): 30031–9. doi:10.1074/jbc.M203811200. PMID 12039962.
- ^ a b Lin HK, Hu YC, Lee DK, Chang C (October 2004). "Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells". Mol. Endocrinol. 18 (10): 2409–23. doi:10.1210/me.2004-0117. PMID 15205473.
- ^ Wang L, Hsu CL, Ni J, Wang PH, Yeh S, Keng P, Chang C (March 2004). "Human Checkpoint Protein hRad9 Functions as a Negative Coregulator To Repress Androgen Receptor Transactivation in Prostate Cancer Cells". Mol. Cell. Biol. 24 (5): 2202–13. doi:10.1128/MCB.24.5.2202-2213.2004. PMC 350564. PMID 14966297. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=350564.
- ^ Rao MA, Cheng H, Quayle AN, Nishitani H, Nelson CC, Rennie PS (December 2002). "RanBPM, a nuclear protein that interacts with and regulates transcriptional activity of androgen receptor and glucocorticoid receptor". J. Biol. Chem. 277 (50): 48020–7. doi:10.1074/jbc.M209741200. PMID 12361945.
- ^ Beitel LK, Elhaji YA, Lumbroso R, Wing SS, Panet-Raymond V, Gottlieb B, Pinsky L, Trifiro MA (August 2002). "Cloning and characterization of an androgen receptor N-terminal-interacting protein with ubiquitin-protein ligase activity". J. Mol. Endocrinol. 29 (1): 41–60. doi:10.1677/jme.0.0290041. PMID 12200228.
- ^ Lu J, Danielsen M (November 1998). "Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein". J. Biol. Chem. 273 (47): 31528–33. doi:10.1074/jbc.273.47.31528. PMID 9813067.
- ^ Yeh S, Miyamoto H, Nishimura K, Kang H, Ludlow J, Hsiao P, Wang C, Su C, Chang C (July 1998). "Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells". Biochem. Biophys. Res. Commun. 248 (2): 361–7. doi:10.1006/bbrc.1998.8974. PMID 9675141.
- ^ Miyamoto H, Rahman M, Takatera H, Kang HY, Yeh S, Chang HC, Nishimura K, Fujimoto N, Chang C (February 2002). "A dominant-negative mutant of androgen receptor coregulator ARA54 inhibits androgen receptor-mediated prostate cancer growth". J. Biol. Chem. 277 (7): 4609–17. doi:10.1074/jbc.M108312200. PMID 11673464.
- ^ Kang HY, Yeh S, Fujimoto N, Chang C (March 1999). "Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor". J. Biol. Chem. 274 (13): 8570–6. doi:10.1074/jbc.274.13.8570. PMID 10085091.
- ^ Moilanen AM, Poukka H, Karvonen U, Häkli M, Jänne OA, Palvimo JJ (September 1998). "Identification of a Novel RING Finger Protein as a Coregulator in Steroid Receptor-Mediated Gene Transcription". Mol. Cell. Biol. 18 (9): 5128–39. PMC 109098. PMID 9710597. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=109098.
- ^ Poukka H, Aarnisalo P, Santti H, Jänne OA, Palvimo JJ (January 2000). "Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms". J. Biol. Chem. 275 (1): 571–9. doi:10.1074/jbc.275.1.571. PMID 10617653.
- ^ Liu Y, Kim BO, Kao C, Jung C, Dalton JT, He JJ (May 2004). "Tip110, the human immunodeficiency virus type 1 (HIV-1) Tat-interacting protein of 110 kDa as a negative regulator of androgen receptor (AR) transcriptional activation". J. Biol. Chem. 279 (21): 21766–73. doi:10.1074/jbc.M314321200. PMID 15031286.
- ^ Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D (January 2002). "The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3". J. Biol. Chem. 277 (2): 1240–8. doi:10.1074/jbc.M108855200. PMID 11707452.
- ^ Hayes SA, Zarnegar M, Sharma M, Yang F, Peehl DM, ten Dijke P, Sun Z (March 2001). "SMAD3 represses androgen receptor-mediated transcription". Cancer Res. 61 (5): 2112–8. PMID 11280774.
- ^ Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C (November 2002). "Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4". J. Biol. Chem. 277 (46): 43749–56. doi:10.1074/jbc.M205603200. PMID 12226080.
- ^ Gobinet J, Auzou G, Nicolas JC, Sultan C, Jalaguier S (December 2001). "Characterization of the interaction between androgen receptor and a new transcriptional inhibitor, SHP". Biochemistry 40 (50): 15369–77. doi:10.1021/bi011384o. PMID 11735420.
- ^ Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M (October 2004). "Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence". Cancer Res. 64 (19): 7156–68. doi:10.1158/0008-5472.CAN-04-1121. PMID 15466214.
- ^ Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL (March 2004). "Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate". Endocr. Relat. Cancer 11 (1): 117–30. doi:10.1677/erc.0.0110117. PMID 15027889.
- ^ Yuan X, Lu ML, Li T, Balk SP (December 2001). "SRY interacts with and negatively regulates androgen receptor transcriptional activity". J. Biol. Chem. 276 (49): 46647–54. doi:10.1074/jbc.M108404200. PMID 11585838.
- ^ Matsuda T, Junicho A, Yamamoto T, Kishi H, Korkmaz K, Saatcioglu F, Fuse H, Muraguchi A (April 2001). "Cross-talk between signal transducer and activator of transcription 3 and androgen receptor signaling in prostate carcinoma cells". Biochem. Biophys. Res. Commun. 283 (1): 179–87. doi:10.1006/bbrc.2001.4758. PMID 11322786.
- ^ Ueda T, Bruchovsky N, Sadar MD (March 2002). "Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways". J. Biol. Chem. 277 (9): 7076–85. doi:10.1074/jbc.M108255200. PMID 11751884.
- ^ Ting HJ, Yeh S, Nishimura K, Chang C (January 2002). "Supervillin associates with androgen receptor and modulates its transcriptional activity". Proc. Natl. Acad. Sci. U.S.A. 99 (2): 661–6. doi:10.1073/pnas.022469899. PMC 117362. PMID 11792840. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=117362.
- ^ Mu X, Chang C (October 2003). "TR2 orphan receptor functions as negative modulator for androgen receptor in prostate cancer cells PC-3". Prostate 57 (2): 129–33. doi:10.1002/pros.10282. PMID 12949936.
- ^ Lee YF, Shyr CR, Thin TH, Lin WJ, Chang C (December 1999). "Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: A unique signaling pathway in the steroid receptor superfamily". Proc. Natl. Acad. Sci. U.S.A. 96 (26): 14724–9. doi:10.1073/pnas.96.26.14724. PMC 24715. PMID 10611280. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=24715.
- ^ Wang X, Yang Y, Guo X, Sampson ER, Hsu CL, Tsai MY, Yeh S, Wu G, Guo Y, Chang C (May 2002). "Suppression of androgen receptor transactivation by Pyk2 via interaction and phosphorylation of the ARA55 coregulator". J. Biol. Chem. 277 (18): 15426–31. doi:10.1074/jbc.M111218200. PMID 11856738.
- ^ Hsiao PW, Chang C (August 1999). "Isolation and characterization of ARA160 as the first androgen receptor N-terminal-associated coactivator in human prostate cells". J. Biol. Chem. 274 (32): 22373–9. doi:10.1074/jbc.274.32.22373. PMID 10428808.
- ^ Miyajima N, Maruyama S, Bohgaki M, Kano S, Shigemura M, Shinohara N, Nonomura K, Hatakeyama S (May 2008). "TRIM68 regulates ligand-dependent transcription of androgen receptor in prostate cancer cells". Cancer Res. 68 (9): 3486–94. doi:10.1158/0008-5472.CAN-07-6059. PMID 18451177.
- ^ Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Jänne OA (July 1999). "Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription". J. Biol. Chem. 274 (27): 19441–6. doi:10.1074/jbc.274.27.19441. PMID 10383460.
- ^ Müller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schüle R (February 2000). "FHL2, a novel tissue-specific coactivator of the androgen receptor". EMBO J. 19 (3): 359–69. doi:10.1093/emboj/19.3.359. PMC 305573. PMID 10654935. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=305573.
- ^ Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP (July 2002). "Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor". Mol. Endocrinol. 16 (7): 1492–501. doi:10.1210/me.16.7.1492. PMID 12089345.
- ^ Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, Choi E, Balk SP, Hollenberg AN (February 2005). "The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists". J. Biol. Chem. 280 (8): 6511–9. doi:10.1074/jbc.M408972200. PMID 15598662.
- ^ Markus SM, Taneja SS, Logan SK, Li W, Ha S, Hittelman AB, Rogatsky I, Garabedian MJ (February 2002). "Identification and Characterization of ART-27, a Novel Coactivator for the Androgen Receptor N Terminus". Mol. Biol. Cell 13 (2): 670–82. doi:10.1091/mbc.01-10-0513. PMC 65658. PMID 11854421. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=65658.
- ^ Sharma M, Li X, Wang Y, Zarnegar M, Huang CY, Palvimo JJ, Lim B, Sun Z (November 2003). "hZimp10 is an androgen receptor co-activator and forms a complex with SUMO-1 at replication foci". EMBO J. 22 (22): 6101–14. doi:10.1093/emboj/cdg585. PMC 275443. PMID 14609956. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=275443.
External links
- GeneReviews/NCBI/NIH/UW entry on Androgen Insensitivity Syndrome
- OMIM entries on Androgen Insensitivity Syndrome
- GeneReviews/NIH/NCBI/UW entry on Spinal and Bulbar Muscular Atrophy, Kennedy's Disease, SBMA, X-Linked Spinal and Bulbar Muscular Atrophy
- OMIM entries on Spinal and Bulbar Muscular Atrophy, Kennedy's Disease, SBMA, X-Linked Spinal and Bulbar Muscular Atrophy
- MeSH Androgen+Receptors
- Brinkmann AO. "Androgen physiology: receptor and metabolic disorders". In Robert McLachlan, editor. Endocrinology of Male Reproduction. Endotext.org. http://www.endotext.org/male/male3/male3.pdf. Retrieved 2008-04-29.
- Gottlieb B (2007-07-24). "The Androgen Receptor Gene Mutations Database Server". McGill University. http://androgendb.mcgill.ca/. Retrieved 2008-04-29.
- Thompson J (2006-09-30). "Molecular Mechanisms of Androgen Receptor Interactions". Helsinki University Biomedical Dissertations No. 80. University of Helsinki. http://ethesis.helsinki.fi/julkaisut/laa/biola/vk/thompson/molecula.pdf. Retrieved 2008-04-29.
Transcription factors and intracellular receptors (1) Basic domains (1.1) Basic leucine zipper (bZIP)Activating transcription factor (AATF, 1, 2, 3, 4, 5, 6, 7) · AP-1 (c-Fos, FOSB, FOSL1, FOSL2, JDP2, c-Jun, JUNB, JUND) · BACH (1, 2) · BATF · BLZF1 · C/EBP (α, β, γ, δ, ε, ζ) · CREB (1, 3, L1) · CREM · DBP · DDIT3 · GABPA · HLF · MAF (B, F, G, K) · NFE (2, L1, L2, L3) · NFIL3 · NRL · NRF (1, 2, 3) · XBP1(1.2) Basic helix-loop-helix (bHLH)ATOH1 · AhR · AHRR · ARNT · ASCL1 · BHLHB2 · BMAL (ARNTL, ARNTL2) · CLOCK · EPAS1 · FIGLA · HAND (1, 2) · HES (5, 6) · HEY (1, 2, L) · HES1 · HIF (1A, 3A) · ID (1, 2, 3, 4) · LYL1 · MESP2 · MXD4 · MYCL1 · MYCN · Myogenic regulatory factors (MyoD, Myogenin, MYF5, MYF6) · Neurogenins (1, 2, 3) · NeuroD (1, 2) · NPAS (1, 2, 3) · OLIG (1, 2) · Pho4 · Scleraxis · SIM (1, 2) · TAL (1, 2) · Twist · USF1(1.3) bHLH-ZIP(1.4) NF-1(1.5) RF-X(1.6) Basic helix-span-helix (bHSH)(2) Zinc finger DNA-binding domains (2.1) Nuclear receptor (Cys4)subfamily 1 (Thyroid hormone (α, β), CAR, FXR, LXR (α, β), PPAR (α, β/δ, γ), PXR, RAR (α, β, γ), ROR (α, β, γ), Rev-ErbA (α, β), VDR)
subfamily 2 (COUP-TF (I, II), Ear-2, HNF4 (α, γ), PNR, RXR (α, β, γ), Testicular receptor (2, 4), TLX)
subfamily 3 (Steroid hormone (Androgen, Estrogen (α, β), Glucocorticoid, Mineralocorticoid, Progesterone), Estrogen related (α, β, γ))
subfamily 4 NUR (NGFIB, NOR1, NURR1) · subfamily 5 (LRH-1, SF1) · subfamily 6 (GCNF) · subfamily 0 (DAX1, SHP)(2.2) Other Cys4(2.3) Cys2His2General transcription factors (TFIIA, TFIIB, TFIID, TFIIE (1, 2), TFIIF (1, 2), TFIIH (1, 2, 4, 2I, 3A, 3C1, 3C2))
ATBF1 · BCL (6, 11A, 11B) · CTCF · E4F1 · EGR (1, 2, 3, 4) · ERV3 · GFI1 · GLI-Krüppel family (1, 2, 3, REST, S2, YY1) · HIC (1, 2) · HIVEP (1, 2, 3) · IKZF (1, 2, 3) · ILF (2, 3) · KLF (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17) · MTF1 · MYT1 · OSR1 · PRDM9 · SALL (1, 2, 3, 4) · SP (1, 2, 4, 7, 8) · TSHZ3 · WT1 · Zbtb7 (7A, 7B) · ZBTB (16, 17, 20, 32, 33, 40) · zinc finger (3, 7, 9, 10, 19, 22, 24, 33B, 34, 35, 41, 43, 44, 51, 74, 143, 146, 148, 165, 202, 217, 219, 238, 239, 259, 267, 268, 281, 295, 300, 318, 330, 346, 350, 365, 366, 384, 423, 451, 452, 471, 593, 638, 644, 649, 655)(2.4) Cys6(2.5) Alternating composition(3) Helix-turn-helix domains (3.1) HomeodomainARX · CDX (1, 2) · CRX · CUTL1 · DBX (1, 2) · DLX (3, 4, 5) · EMX2 · EN (1, 2) · FHL (1, 2, 3) · HESX1 · HHEX · HLX · Homeobox (A1, A2, A3, A4, A5, A7, A9, A10, A11, A13, B1, B2, B3, B4, B5, B6, B7, B8, B9, B13, C4, C5, C6, C8, C9, C10, C11, C12, C13, D1, D3, D4, D8, D9, D10, D11, D12, D13) · HOPX · IRX (1, 2, 3, 4, 5, 6, MKX) · LMX (1A, 1B) · MEIS (1, 2) · MEOX2 · MNX1 · MSX (1, 2) · NANOG · NKX (2-1, 2-2, 2-3, 2-5, 3-1, 3-2, 6-1, 6-2) · NOBOX · PBX (1, 2, 3) · PHF (1, 3, 6, 8, 10, 16, 17, 20, 21A) · PHOX (2A, 2B) · PITX (1, 2, 3) · POU domain (PIT-1, BRN-3: A, B, C, Octamer transcription factor: 1, 2, 3/4, 6, 7, 11) · OTX (1, 2) · PDX1 · SATB2 · SHOX2 · VAX1 · ZEB (1, 2)(3.2) Paired box(3.3) Fork head / winged helix(3.4) Heat Shock Factors(3.5) Tryptophan clusters(3.6) TEA domain(4) β-Scaffold factors with minor groove contacts (4.1) Rel homology region(4.2) STAT(4.3) p53(4.4) MADS box(4.6) TATA binding proteins(4.7) High-mobility group(4.10) Cold-shock domainCSDA, YBX1(4.11) Runt(0) Other transcription factors (0.2) HMGI(Y)(0.3) Pocket domain(0.6) MiscellaneousEstrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: FulvestrantCategories:- Human proteins
- Intracellular receptors
- Transcription factors
Wikimedia Foundation. 2010.