- Chimeric antigen receptor
-
Artificial T cell receptors (also known as chimeric T cell receptors, chimeric immunoreceptors, chimeric antigen receptors (CARs)) are engineered receptors, which graft an arbitrary specificity onto an immune effector cell. Typically, these receptors are used to graft the specificity of a monoclonal antibody onto a T cell; with transfer of their coding sequence facilitated by retroviral vectors. In this way, a large number of cancer-specific T cells can be generated for use as adoptive cell therapy. [1]. Phase I clinical studies of this approach show efficacy.
Contents
Anatomy of Chimeric Antigen Receptors
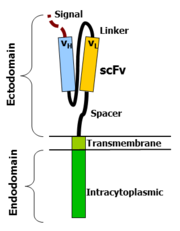
The most common form of these molecules are fusions of single-chain Variable fragments (scFv) derived from monoclonal antibodies, fused to CD3-zeta transmembrane and endodomain. Such molecules result in the transmission of a zeta signal in response to recognition by the scFv of its target. An example of such a construct is 14g2a-Zeta, which is a fusion of a scFv derived from hybridoma 14g2a (which recognizes disialoganglioside GD2). When T cells express this molecule (usually achieved by oncoretroviral vector transduction), they recognize and kill target cells that express GD2 (e.g. neuroblastoma cells). To target malignant B cells, investigators have redirected the specificity of T cells using a chimeric immunoreceptor specific for the B-lineage molecule, CD19.
The variable portions of an immunoglobulin heavy and light chain are fused by a flexible linker to form a scFv. This scFv is preceded by a signal peptide to direct the nascent protein to the endoplasmic reticulum and subsequent surface expression (this is cleaved). A flexible spacer allows to the scFv to orient in different directions to enable antigen binding. The transmembrane domain is a typical hydrophobic alpha helix usually derived from the original molecule of the signalling endodomain which protrudes into the cell and transmits the desired signal.
The fact that these molecules actually work is at first glance surprising. At second glance one remembers that type I proteins are in fact two protein domains linked by a transmembrane alpha helix in between. The cell membrane lipid bilayer, through which the transmembrane domain passes, acts to isolate the inside portion (endodomain) from the external portion (ectodomain). It is not so surprising hence that attaching an ectodomain from one protein to an endodomain of another protein results in a molecule that combines the recognition of the former to the signal of the latter.
To more completely appreciate the engineering work that goes into these molecules, the different components will be discussed separately.Ectodomain - signal peptide
A signal peptide directs the nascent protein into the endoplasmic reticulum. This is essential if the receptor is to be glycosylated and anchored in the cell membrane. Any eukaryotic signal peptide sequence usually works fine. Generally, the signal peptide natively attached to the amino-terminal most component is used (e.g. in a scFv with orientation light-chain - linker - heavy chain, the native signal of the light-chain is used
Ectodomain - antigen recognition region
The antigen recognition domain is usually an scFv. There are however many alternatives. An antigen recognition domain from native TCR alpha and beta single chains have been described, as have simple ectodomains (e.g. CD4 ectodomain to recognize HIV infected cells) and more exotic recognition components such as a linked cytokine (which leads to recognition of cells bearing the cytokine receptor). In fact almost anything that binds a given target with high affinity can be used as an antigen recognition region.
Ectodomain - spacer
A spacer region links the antigen binding domain to the transmembrane domain. It should be flexible enough to allow the antigen binding domain to orient in different directions to facilitate antigen recognition. The simplest form is the hinge region from IgG1. Alternatives include the CH2CH3 region of immunoglobulin and portions of CD3. For most scFv based constructs, the IgG1 hinge suffices. However the best spacer often has to be determined empirically.
Transmembrane domain
This is a hydrophobic alpha helix that spans the membrane. Generally, the transmembrane domain from the most membrane proximal component of the endodomain is used. Interestingly, using the CD3-zeta transmembrane domain may result in incorporation of the artificial TCR into the native TCR a factor that is dependent on the presence of the native CD3-zeta transmembrane charged aspartic acid residue [2] . Different transmembrane domains result in different receptor stability. The CD28 transmembrane domain results in a brightly expressed, stable receptor.
Endodomain
This is the business-end of the receptor. After antigen recognition, receptors cluster and a signal is transmitted to the cell. The most commonly used endodomain component is CD3-zeta which contains 3 ITAMs. This transmits an activation signal to the T cell after antigen is bound. CD3-zeta may not provide a fully competent activation signal and additional co-stimulatory signaling is needed. For example, chimeric CD28 and OX40 can be used with CD3-Zeta to transmit a proliferative / survival signal, or all three can be used together.
History of Chimeric Antigen Receptors
http://www.nejm.org/doi/full/10.1056/NEJMe1106965
Clinical Studies testing Chimeric Antigen Receptors
References
- ^ Pule, M; Finney H, Lawson A (2003). "Artificial T-cell receptors". Cytotherapy 5 (3): 211–26. doi:10.1080/14653240310001488. PMID 12850789.
- ^ Bridgeman, JS; Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE (2010). "The Optimal Antigen Response of Chimeric Antigen Receptors Harbouring the CD3zeta Transmembrane Domain Is Dependent Upon Incorporation of the Receptor Into the Endogenous TCR/CD3 Complex". Journal of Immunology 184 (12): 6938–49. doi:10.4049/jimmunol.0901766. PMID 20483753.
- Rossig, C; et al. (Oct 15 2001). "Targeting of GD2 positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes". Int J Cancer 94 (2): 228–36. doi:10.1002/ijc.1457. PMID 11668503.
Categories:- Immune system
- T cells
Wikimedia Foundation. 2010.