- African trypanosomiasis
-
For other uses, see Sleeping sickness (disambiguation).For "Sleepy sickness", see Encephalitis lethargica.
African trypanosomiasis Classification and external resources 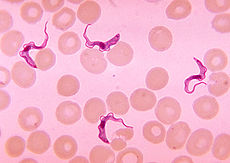
Trypanosoma forms in a blood smear.ICD-10 B56 ICD-9 086.5 DiseasesDB 29277 13400 MedlinePlus 001362 eMedicine med/2140 Human African trypanosomiasis, sleeping sickness,[1] African lethargy,[1] or Congo trypanosomiasis[1] is a parasitic disease of people and animals, caused by protozoa of the species Trypanosoma brucei and transmitted by the tsetse fly.[2] The disease is endemic in some regions of sub-Saharan Africa, covering about 37 countries and 60 million people. It is estimated that 50,000 to 70,000 people are currently infected, the number having declined somewhat in recent years.[3] The number of reported cases was below 10,000 in 2009, the first time in 50 years.[4] It is believed that many cases go unreported. About 48,000 people died of it in 2008.[5] Four major epidemics have occurred in recent history: one from 1896–1906 primarily in Uganda and the Congo Basin, two epidemics in 1920 and 1970 in several African countries, and a recent 2008 epidemic in Uganda.[6]
Contents
Signs and symptoms
African trypanosomiasis symptoms occur in two stages. The first stage is known as the haemolymphatic phase and is characterized by fever, headaches, joint pains, and itching. Invasion of the circulatory and lymphatic system by the parasites is associated with severe swelling of lymph nodes, often to tremendous sizes. Winterbottom's sign, the tell-tale swollen lymph nodes along the back of the neck, may appear. If left untreated, the disease overcomes the host's defenses and can cause more extensive damage, broadening symptoms to include anemia, endocrine, cardiac, and kidney dysfunctions. The second stage, called the neurological phase, begins when the parasite invades the central nervous system by passing through the blood-brain barrier. The term 'sleeping sickness' comes from the symptoms of the neurological phase. The symptoms include confusion, reduced coordination, and disruption of the sleep cycle, with bouts of fatigue punctuated with manic periods leading to daytime slumber and night-time insomnia[citation needed]. Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma and death. Damage caused in the neurological phase is irreversible.[6]
Tryptophol is a chemical compound that induces sleep in humans. It is produced by the trypanosomal parasite in sleeping sickness.[7]
Life cycle
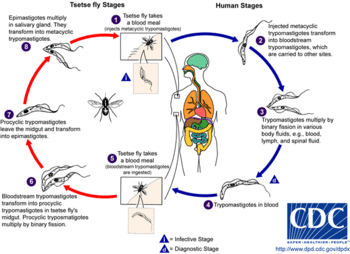
The tsetse fly (genus Glossina) is a large, brown biting fly that serves as both a host and vector for the Trypanosome parasites. While taking blood from a mammalian host, an infected tsetse fly injects metacyclic trypomastigotes into skin tissue. From the bite, parasites first enter the lymphatic system and then pass into the bloodstream. Inside the mammalian host, they transform into bloodstream trypomastigotes, and are carried to other sites throughout the body, reach other blood fluids (e.g., lymph, spinal fluid), and continue to replicate by binary fission.
The entire life cycle of African trypanosomes is represented by extracellular stages. A tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host. In the fly's midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission, leave the midgut, and transform into epimastigotes. The epimastigotes reach the fly's salivary glands and continue multiplication by binary fission.
The entire life cycle of the fly takes approximately 3 weeks.
In addition to the bite of the tsetse fly, the disease can be transmitted in the following ways:
- Mother to child infection: the trypanosome can sometimes cross the placenta and infect the fetus.[8]
- Laboratories: accidental infections, for example, through the handling of blood of an infected person and organ transplantation, although this is uncommon.
- Blood transfusion
- Sexual contact (This may be possible)[9]
Diagnosis
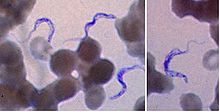 Two areas from a blood smear from a patient with African trypanosomiasis. Thin blood smear stained with Giemsa. Typical trypomastigote stages (the only stages found in patients), with a posterior kinetoplast, a centrally located nucleus, an undulating membrane, and an anterior flagellum. The two Trypanosoma brucei subspecies that cause human trypanosomiasis, T. b. gambiense and T. b. rhodesiense, are indistinguishable morphologically. The trypanosomes' length range is 14 to 33 µm, Source: CDC
Two areas from a blood smear from a patient with African trypanosomiasis. Thin blood smear stained with Giemsa. Typical trypomastigote stages (the only stages found in patients), with a posterior kinetoplast, a centrally located nucleus, an undulating membrane, and an anterior flagellum. The two Trypanosoma brucei subspecies that cause human trypanosomiasis, T. b. gambiense and T. b. rhodesiense, are indistinguishable morphologically. The trypanosomes' length range is 14 to 33 µm, Source: CDC
The gold standard for diagnosis is identification of trypanosomes in a patient sample by microscopic examination. Patient samples that can be used for diagnosis include chancre fluid, lymph node aspirates, blood, bone marrow, and, during the neurological stage, cerebrospinal fluid. Detection of trypanosome-specific antibodies can be used for diagnosis, but the sensitivity and specificity of these methods are too variable to be used alone for clinical diagnosis. Further, seroconversion occurs after the onset of clinical symptoms during a T. b. rhodesiense infection, and therefore is of limited diagnostic use[citation needed].
Trypanosomes can be detected from patient samples using two different preparations. A wet preparation can be used to look for the motile trypanosomes. Alternatively, a fixed (dried) smear can be stained with Giemsa (or Field) and examined. Often the parasite is in relatively low abundance in the sample, so techniques to concentrate the parasites can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the buffy coat; mini anion-exchange/centrifugation; and the Quantitative Buffy Coat (QBC) technique. For other samples such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment[citation needed].
Three serological tests are also available for detection of the parasite: the micro-CATT, wb-CATT, and wb-LATEX. The first uses dried blood while the other two use whole blood samples. A 2002 study found the wb-CATT to be the most efficient for diagnosis, while the wb-LATEX is a better exam for situations where greater sensitivity is required.[10]
Prevention
See also: Tsetse_fly#Control_techniquesTwo alternative strategies have been used in the attempts to reduce the African trypanosomiases. The primary method focuses on the eradication of the tsetse fly, which disrupts transmission rates by reducing the number of flies. Instances of sleeping sickness are being reduced by the use of the sterile insect technique. The second tactic is primarily medical or veterinary and tries to reduce spread of the parasite by monitoring, prophylaxis, treatment, and surveillance to reduce the number of people/animals that carry the disease[citation needed].
Regular active surveillance, involving detection and prompt treatment of new infections, and tsetse fly control is the backbone of the strategy used to control sleeping sickness. Systematic screening of at-risk communities is the best approach, because case-by-case screening is not practical in endemic regions. Systematic screening may be in the form of mobile clinics or fixed screening centres where teams travel daily to areas of high infection rates. Such screening efforts are important because early symptoms are not evident or serious enough to warrant patients with gambiense disease to seek medical attention, particularly in very remote areas. Also, diagnosis of the disease is difficult and health workers may not associate such general symptoms with trypanosomiasis. Systematic screening allows early-stage disease to be detected and treated before the disease progresses, and removes the potential human reservoir.[11] There is a single case report of sexual transmission of West African sleeping sickness,[12] but this is not believed to be an important route of transmission.
Treatment
First line, first stage
The current standard treatment for first stage (haemolymphatic) disease is:
- Intravenous or intramuscular pentamidine (for T.b. gambiense); or
- Intravenous suramin (for T.b. rhodesiense)
The drug Eflornithine — previously used only as an alternative treatment for sleeping sickness due to its labour-intensive administration — was found to be safe and effective as a first-line treatment for the disease in 2008, according to the Science and Development Network's Sub-Saharan Africa news updates. [1]. Researchers tracked over 1,000 adults and children at a centre in Ibba, Southern Sudan—the first use of eflornithine on a large scale— and it was highly effective in treating the issue.
According to a treatment study of Trypanosoma gambiense caused human African trypanosomiasis, use of eflornithine (DMFO) resulted in fewer adverse events than treatment with melarsoprol.[13]
First line, second stage
The current standard treatment for second stage (neurological phase) disease is:
- Intravenous melarsoprol 2.2 mg/kg daily for 12 consecutive days.[14]
Alternative first line therapies include:
- Intravenous melarsoprol 0.6 mg/kg on day 1, 1.2 mg/kg IV melarsoprol on day 2, and 1.2 mg/kg/day IV melarsoprol combined with oral 7.5 mg/kg nifurtimox twice a day on days 3 to 10;[15] or
- Intravenous eflornithine 50 mg/kg every six hours for 14 days.[16]
Combination therapy with eflornithine and nifurtimox is safer and easier than treatment with eflornithine alone, and appears to be equally or more effective. It has been recommended as first-line treatment for second stage T. b. gambiensis disease.[17]
Resistant disease
In areas with melarsoprol resistance or in patients who have relapsed after melarsoprol monotherapy, the treatment should be:
- melarsoprol and nifurtimox, or
- eflornithine
Outdated protocols
The following traditional regimens should no longer be used:
- (old "standard" 26-day melarsoprol therapy) Intravenous melarsoprol therapy (3 series of 3.6 mg/kg/day intravenously for 3 days, with 7-day breaks between the series) (this regimen is less convenient and patients are less likely to complete therapy);[18]
- (incremental melarsoprol therapy) 10-day incremental-dose melarsoprol therapy (0.6 mg/kg iv on day 1, 1.2 mg/kg iv on day 2, and 1.8 mg/kg iv on days 3–10) (previously thought to reduce the risk of treatment-induced encephalopathy, but now known to be associated with an increased risk of relapse and a higher incidence of encephalopathy);[15][18]
After successful treatment, all patients should be followed up for two years with lumbar punctures every six months to look for relapse.
Epidemiology
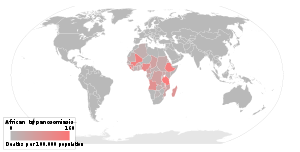
The disease is found in two forms, depending on the parasite, either Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense. Humans are the main reservoir for Trypanosoma brucei gambiense, but this species can also be found in pigs and other animals. Wild game animals and cattle are the main reservoir of T. b. rhodesiense. T. b. gambiense is found in central and western Africa; it causes a chronic condition that can remain in a passive phase for months or years before symptoms emerge. T. b. rhodesiense is the acute form of the disease, but has a much more limited geographic range. It is found in southern and eastern Africa and symptoms of infection emerges in a few weeks and is more virulent and faster developing than T. b. gambiense. According to recent estimates, the disability adjusted life years (9 to 10 years) (DALYs) lost due to sleeping sickness are 2.0 million.[20] Recent estimates indicate that over 60 million people living in some 250 locations are at risk of contracting the disease, and there were under 10,000 cases reported in 2009 according to WHO figures which represents a huge decrease from the estimated 300,000 new cases in 1998.[21] The disease has been recorded as occurring in 36 countries, all in sub-Saharan Africa. It is endemic in southeast Uganda and western Kenya, and killed more than 48,000 Africans in 2008.[6]
Horse-flies (Tabanidae) and stable flies (Muscidae) possibly play a role in transmission of nagana (the animal form of sleeping sickness) and the human disease form.[22]
History
The condition has been present in Africa since at least the 14th century, and probably for thousands of years before that. Because there was a lack of travel between indigenous people, sleeping sickness in humans had been limited to isolated pockets. This changed once Arab slave traders entered central Africa from the east, following the Congo River, bringing parasites along. Gambian sleeping sickness travelled up the Congo River, then further eastwards. In 1901 a devastating epidemic had erupted in Uganda, killing more than 250,000 people,[23] about two-thirds of the population in the affected lake-shore areas. According to The Cambridge history of Africa, "It has been estimated that up to half the people died of sleeping-sickness and smallpox in the lands on either bank of the lower river Congo."[24]
The causative agent and vector were identified in 1903 by David Bruce, and the differentiation between the subspecies of the protozoa made in 1910. The first effective treatment, atoxyl, an arsenic-based drug developed by Paul Ehrlich and Kiyoshi Shiga, was introduced in 1910, but blindness was a serious side effect. Numerous drugs designed to treat the disease have been introduced since then[citation needed].
Suramin was introduced in 1920 to treat the first stage of the disease. By 1922, Suramin was generally combined with Tryparsamide (another pentavalent organo-arsenic drug) in the treatment of the second stage of the gambiense form. It was used during the grand epidemic in West and Central Africa in millions of people and was the mainstay of therapy until 1969[citation needed].
Pentamidine, a highly effective drug for the first stage of the disease, has been used since 1939. During the fifties, it was widely used as a prophylactic agent in Western Africa, leading to a sharp decline in infection rates. At the time, it was thought that eradication of the disease was at hand[citation needed].
The organo-arsenical melarsoprol (Arsobal) was developed in the 1940s, and is effective for patients with second stage sleeping sickness. However, 3-10% of those injected have reactive encephalopathy (convulsions, progressive coma, or psychotic reactions), and 10-70% of such cases result in death; it can cause brain damage in those who survive the encephalopathy. However, due to its effectiveness, melarsoprol is still used today. Resistance to melarsoprol is increasing, and combination therapy with nifurtimox is currently under research[citation needed].
Eflornithine (difluoromethylornithine or DFMO), the most modern treatment, was developed in the 1970s by Albert Sjoerdsmanot and underwent clinical trials in the 1980s. The drug was approved by the United States Food and Drug Administration in 1990, but Aventis, the company responsible for its manufacture, halted production in 1999. In 2001, however, Aventis, in association with Médecins Sans Frontières and the World Health Organization, signed a long-term agreement to manufacture and donate the drug[citation needed].
Research
The genome of the parasite has been sequenced and several proteins have been identified as potential targets for drug treatment. Analysis of the genome also revealed the reason why generating a vaccine for this disease has been so difficult. T. brucei has over 800 genes that make proteins the parasite "mixes and matches" to evade immune system detection.[25]
Recent findings indicate that the parasite is unable to survive in the bloodstream without its flagellum. This insight gives researchers a new angle with which to attack the parasite.[26]
A new treatment based on a truncated version of the apolipoprotein L-1 of high density lipoprotein and a single-domain antibody has recently been found to work in mice, but has not been tested in humans.[27]
The cover story of the August 25, 2006 issue of the journal Cell describes an advance in understanding how Trypanosomes escape the immune system. Dr. Lee Soo Hee and colleagues, working at Johns Hopkins investigated the pathway by which Trypanosomes make myristate, a 14-carbon length fatty acid. Myristate is a component of the variant surface glycoprotein (VSG), the molecule that makes up the trypanosome's outer layer. This outer surface coat of VSG is vital to the trypanosome's ability to avoid destruction by the host's immune system. Dr. Lee and colleagues discovered trypanosomes use a novel fatty acid synthesis pathway involving fatty acid elongases to make myristate and other fatty acids.
An international research team working in the Democratic Republic of the Congo, Southern Sudan, and Angola involving Immtech International and University of North Carolina at Chapel Hill have completed a Phase IIb clinical trial and began a Phase III trial in 2005 testing the efficacy of the first oral treatment for sleeping sickness, pafuramidine (DB289).[28][29] Trypanosomiasis vaccines are undergoing research.
Two independent variants of the APOL1 gene found in African haplotypes carrying signatures of natural selection have been shown to confer protection against the acute version of sleeping sickness caused by Trypanosoma brucei rhodesiense while at the same time increasing risk of kidney disease when inherited from both parents.[30]
Synthetic and computer based approaches are used for the development of newer anti-trypanosomal analogues with improved efficacy and oral bioavailability.[31]
See also
- Human trypanosomiasis
- Drugs for Neglected Diseases Initiative
- David Bruce (microbiologist)
- Sleep disorder
- Chagas disease (American trypanosomiasis), another human tropical disease caused by trypanosomes
- Nagana (Animal African Trypanosomiasis)
- Tsetse fly
- G.D. Hale Carpenter joined the London School of Hygiene and Tropical Medicine, and took the DM in 1913 with a dissertation on the tsetse fly (Glossina palpalis) and sleeping sickness. He published A Naturalist on Lake Victoria, with an Account of Sleeping Sickness and the Tse-tse Fly; 1920. T.F. Unwin Ltd, London; Biodiversity Archive
References
- ^ a b c Robinson, Victor, Ph.C., M.D. (editor) (1939). "African Lethargy, Sleeping Sickness, or Congo trypanosomiasis; Trypanosoma gambiense". The Modern Home Physician, A New Encyclopedia of Medical Knowledge. WM. H. Wise & Company (New York)., pages 20-21.
- ^ "Sleeping sickness," Medline Plus, retrieved May 28, 2008.
- ^ WHO Media centre (2006). Fact sheet N°259: African trypanosomiasis or sleeping sickness. http://www.who.int/mediacentre/factsheets/fs259/en/.
- ^ "African trypanosomiasis (sleeping sickness),"WHO, retrieved May 29, 2011.
- ^ "New treatments raise hope of cutting sleeping sickness deaths". The Guardian. May 15, 2009.
- ^ a b c "Uganda: Sleeping Sickness Reaching Alarming Levels," New Vision, May 11, 2008.
- ^ Rapid distribution of tryptophol (3-indole ethanol) to the brain and other tissues. E M Cornford, W D Bocash, L D Braun, P D Crane, W H Oldendorf and A J MacInnis, J Clin Invest. 1979 June; 63(6), pp. 1241–1248, doi:10.1172/JCI109419, PMC PMC372073
- ^ Olowe SA (1975). "A case of congenital trypanosomiasis in Lagos". Trans. R. Soc. Trop. Med. Hyg. 69 (1): 57–9. doi:10.1016/0035-9203(75)90011-5. PMID 1170654.
- ^ Rocha G, Martins A, Gama G, Brandão F, Atouguia J (January 2004). "Possible cases of sexual and congenital transmission of sleeping sickness". Lancet 363 (9404): 247. doi:10.1016/S0140-6736(03)15345-7. PMID 14738812.
- ^ Truc P, Lejon V, Magnus E, et al. (2002). "Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa". Bull. World Health Organ. 80 (11): 882–6. PMC 2567684. PMID 12481210. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0042-96862002001100008&lng=en&nrm=iso&tlng=en. Retrieved 2009-03-16.
- ^ "Strategic Direction for African Trypanosomiasis Research". Special Programme for Research and Training in Tropical Diseases. http://www.who.int/tdr/diseases/tryp/direction.htm. Retrieved 2006-03-01.
- ^ Rocha G, Martins A, Gama G, Brandão F, Atouguia J (2004). "Possible cases of sexual and congenital transmission of sleeping sickness". Lancet 363 (9404): 247. doi:10.1016/S0140-6736(03)15345-7. PMID 14738812.
- ^ Chappuis F, Udayraj N, Stietenroth K, Meussen A, Bovier PA (2005). "Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis". Clin. Infect. Dis. 41 (5): 748–51. doi:10.1086/432576. PMID 16080099.
- ^ Burri, C; Nkunku, S; Merolle, A; Smith, T; Blum, J; Brun, R (2000). "Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial". Lancet 355 (9213): 1419–25. doi:10.1016/S0140-6736(00)02141-3. PMID 10791526.
- ^ a b Bisser S, N'Siesi FX, Lejon V, et al. (2007). "Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness". J. Infect. Dis. 195 (3): 322–9. doi:10.1086/510534. PMID 17205469.
- ^ van Nieuwenhove S, Schechter PJ, Declercq J, et al. (1985). "Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (DL-alfa-difluoromethyl ornithine) an inhibitor of ornithine decarboxylase: first field trial". Trans R Soc Trop Med Hyg 79 (5): 692–8. doi:10.1016/0035-9203(85)90195-6. PMID 3938090.
- ^ Priotto G, Kasparian S, Mutombo W, et al. (July 2009). "Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial". Lancet 374 (9683): 56–64. doi:10.1016/S0140-6736(09)61117-X. PMID 19559476.
- ^ a b Pepin J, Mpia B (2006). "Randomized controlled trial of three regimens of melarsoprol in the treatment of Trypanosoma brucei gambiense trypanosomiasis". Trans R Soc Trop Med Hyg 100 (5): 437–41. doi:10.1016/j.trstmh.2005.03.017. PMID 16483622.
- ^ WHO mortality and health data and statistics, accessed Feb 10, 2009.
- ^ World Health Organization (Geneva) (2000). World Health Report 2000: Health Systems Improving Performance. http://www.who.int/tdr/diseases/tryp/direction.htm#Refs.
- ^ WHO Expert Committee on Control and Surveillance of African trypanosomiasis (Geneva) (1998). WHO Technical Report Series,No.881. http://www.who.int/tdr/diseases/tryp/direction.htm#Refs.
- ^ Cherenet T, Sani RA, Panandam JM, Nadzr S, Speybroeck N, van den Bossche P (2004). "Seasonal prevalence of bovine trypanosomosis in a tsetse-infested zone and a tsetse-free zone of the Amhara Region, north-west Ethiopia". The Onderstepoort journal of veterinary research 71 (4): 307–312. PMID 15732457.
- ^ Reanalyzing the 1900–1920 Sleeping Sickness Epidemic in Uganda. Centers for Disease Control and Prevention (CDC).
- ^ "The Cambridge history of Africa: From the earliest times to c. 500 BC". John D. Fage (1982). Cambridge University Press. p.748. ISBN 0-521-22803-4
- ^ Berriman M, Ghedin E, Hertz-Fowler C, et al. (2005). "The genome of the African trypanosome Trypanosoma brucei". Science 309 (5733): 416–22. Bibcode 2005Sci...309..416B. doi:10.1126/science.1112642. PMID 16020726. http://www.sciencemag.org/cgi/content/full/309/5733/416.
- ^ "African Sleeping Sickness Breakthrough". http://domino.lancs.ac.uk/info/LUNews.nsf/I/448E635736B6B25A8025714700317FD1. Retrieved April 7, 2006.
- ^ New Scientist, 25 Aug. 2007, pp. 35-7
- ^ Williamson, David (August 25, 2005). "Compound might defeat African sleeping sickness, clinical trial beginning this month". University of North Carolina. http://www.america.gov/st/washfile-english/2005/August/20050826160501cmretrop0.7327387.html.
- ^ Staff (September 15, 2005). "Clinical Trials Update". Genetic Engineering News. p. 5.
- ^ Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. (Jul 2010). "Association of Trypanolytic ApoL1 Variants with Kidney Disease in African-Americans". Science 329 (5993): 841–845. doi:10.1126/science.1193032. PMC 2980843. PMID 20647424. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2980843.
- ^ Paliwal SK, Verma AN, Paliwal S (2011 pmid = 21886894). "Neglected Disease – African Sleeping Sickness: Recent Synthetic and Modeling Advances". Sci. Pharm. 79: 329–428. doi:10.3797/scipharm.1012-08. PMC 316337. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=316337.
External links
- Sleeping sickness information page at Médecins Sans Frontières
- Drugs for Neglected Diseases Initiative
- Medecins Sans Frontieres' Eflornithine press release, 2001
- Links to pictures of Sleeping Sickness (Hardin MD/ University of Iowa)
- The Sandler Center for Basic Research in Parasitic Diseases, University of California, San Francisco.
- Kids For World Health
- Eflornithine 'safe as first-line sleeping sickness treatment'
- Science without Frontiers
- Amaro RE, Swift RV, McCammon JA (2007). "Functional and structural insights revealed by molecular dynamics simulations of an essential RNA editing ligase in Trypanosoma brucei". PLoS Negl Trop Dis 1 (2): e68. doi:10.1371/journal.pntd.0000068. PMC 2100368. PMID 18060084. http://dx.plos.org/10.1371/journal.pntd.0000068. "Flash Video doi:10.4016/5585.01"
Infectious diseases – Parasitic disease: protozoan infection: Excavata (A06–A07, B55–B57, 007, 085–086) Discicristata TrypanosomatidaLeishmania major/L. mexicana/L. aethiopica/L. tropica (Cutaneous leishmaniasis) · L. braziliensis (Mucocutaneous leishmaniasis) · L. donovani/infantum (Visceral leishmaniasis)SchizopyrenidaTrichozoa TrichomonadidaDiseases of poverty Diseases of poverty Neglected diseases Cholera · Chagas disease · African Sleeping Sickness · Schistosomiasis · Guinea worm · River blindness · LeishmaniasisMiscellaneous Categories:- Health in Africa
- Euglenozoa
- Sleep disorders
- Protozoal diseases
- Tropical diseases
- Neglected diseases
- Insect-borne diseases
Wikimedia Foundation. 2010.