- Organogallium chemistry
-
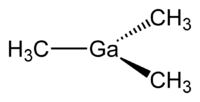
Organogallium chemistry is the chemistry of organometallic compounds containing a carbon to gallium (Ga) chemical bond. Despite their high toxicity organogallium compounds have some use in organic synthesis. The compound trimethylgallium is of some relevance to MOCVD as a precursor to gallium arsenide:
- Ga(CH3)3 + AsH3 → GaAs + 3CH4
with arsine at 700 °C. Gallium trichloride is an important reagent for the introduction of gallium into organic compounds.
The main gallium oxidation state is Ga(III) as in all higher group 13 elements notably aluminium. [1] [2]
Contents
Organogallium(III) chemistry
Compounds of the type R3Ga are monomeric. Lewis acidity decreases in the order Al > Ga > In and as a result organogallium compound do not form bridged dimers as organoaluminum compounds do. Organogallium compounds are also less reactive then organoaluminum compounds.
Organogallium compounds can be synthesized by transmetallation, for example the reaction of gallium metal with dimethylmercury:
- 2Ga + 2Me2Hg → 2Me3Ga + 3 Hg
or via organolithium compounds or Grignards:
- GaCl3 + 3MeMgBr → Me3Ga + 3MgBrCl
The electron-deficient nature of gallium can be removed by complex formation, for example
- Me2GaCl + NH3 → [Me2Ga(NH3)Cl]+Cl-
Pi complex formation with alkynes is also known.
Organogallium compounds are reagents or intermediates in several classes of organic reactions:
- Barbier-type reactions with elemental gallium, allylic substrates and carbonyl compounds
- Carbometallation (carbogallation) reactions [3]
Higher group 13 organometallic chemistry
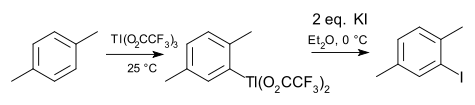
The chemistry of organoindium (In) and organothallium (Tl) compounds parallel that of organogallium in many regards. Indium and thallium in oxidation state +1 are more common, for example the metallocenes cyclopentadienylindium(I) and cyclopentadienyl thallium. Trimethylindium is important in the semiconductor industry. A special thallium feature is electrophilic thallation of arene compounds, reminiscent of mercuration (the group 12 neighbor). A common reagent for this purpose is thalium(III) trifluoroacetate. The intermediate arylthallium bisfluoroacetate can be isolated and converted to an aryl halide, aryl cyanide, aryl thiol or nitroarene. An example is the iodation of para-xylene [4].
See also
- Chemical bonds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ C. Elschenbroich, A. Salzer Organometallics : A Concise Introduction (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ Chemistry of aluminium, gallium, indium, and thallium Anthony John Downs (Ed.) ISBN: 978-0-7514-0103-5 1993
- ^ GaCl3 in Organic Synthesis Ryo Amemiya and Masahiko Yamaguchi Eur. J. Org. Chem. 2005, 5145–5150 doi:10.1002/ejoc.200500512
- ^ Organic Syntheses, Coll. Vol. 6, p.709 (1988); Vol. 55, p.70 (1976). Link
Categories: Gallium compounds | Organometallic compounds
Wikimedia Foundation. 2010.