- Fluphenazine
-
Fluphenazine 
Systematic (IUPAC) name 2-[4-[3-[2-(trifluoromethyl)-10H-phenothiazin-10-yl]propyl]piperazin-1-yl]ethanol Clinical data AHFS/Drugs.com monograph MedlinePlus a682172 Pregnancy cat. C(US) Legal status ℞ Prescription only Routes oral, IM, decanoate Pharmacokinetic data Bioavailability 40% - 50% Metabolism Hepatic Half-life 15 to 30 hours Excretion bile/feces Identifiers CAS number 69-23-8 
ATC code N05AB02 PubChem CID 3372 IUPHAR ligand 204 DrugBank DB00623 ChemSpider 3255 
UNII S79426A41Z 
KEGG D07977 
ChEBI CHEBI:5123 
ChEMBL CHEMBL726 
Chemical data Formula C22H26F3N3OS Mol. mass 437.523 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Fluphenazine is a typical antipsychotic drug used for the treatment of psychoses such as schizophrenia and acute manic phases of bipolar disorder. It belongs to the piperazine class of phenothiazines.
Its main use is as a long acting injection given once every two or three weeks to people with schizophrenia who suffer frequent relapses of illness. Its side effect profile is similar to haloperidol, namely predominantly dopamine-blocking effects which give rise to akathisia, parkinsonism and tremor. Long term side effects include the potentially irreversible tardive dyskinesia and the potentially fatal neuroleptic malignant syndrome.
Contents
Brand names
Fluphenazine decanoate: Modecate, Prolixin Decanoate, Dapotum D, Anatensol, Fludecate, Sinqualone Deconoate Fluphenazine enanthate: Dapotum Injektion, Flunanthate, Moditen Enanthate Injection, Sinqualone Enanthate Fluphenazine hydrochloride: Prolixin, Permitil, Dapotum, Lyogen, Moditen, Omca, Sediten, Selecten, Sevinol, Sinqualone, Trancin flucate
Pharmacokinetics
Fluphenazine has an incomplete oral bioavailability of 40% to 50% (due to extensive first pass metabolization in the liver). Its half life is 15 to 30 hours.
Dosing
12.5 mg of fluphenazine decanoate is roughly equivalent to 100 mg of zuclopenthixol decanoate or 20 mg of flupentixol decanoate.
Side effects
Further information: Typical antipsychoticNotable side effects include akathisia, extrapyramidal side effects, including tardive dyskinesia and Rabbit syndrome. The frequency and severity of extrapyramidal side effects are direct proportional to the dose given and the duration of treatment. Mostly notably, although rare, it can cause hypothermia because it inhibits ability to shiver.
Sedative, allergic-toxic and anticholinergic/sympatholytic side effects are less likely to occur compared with chlorpromazine. The direct deposition of fluphenazine in the cornea and retina has so far not been reported.
Neuroleptic malignant syndrome, although rare, is a potentially lethal side effect of all antipsychotics.
Chemistry
Fluphenazine (4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]-1-piperazineethanol), is synthesized by any of the methods described already for the preparation of trifluoperazine and related antipsychotics.
- G.E. Ullyot, U.S. Patent 3,058,979 (1962).
- R.C. Merril, H.L. Yale, U.S. Patent 3,394,131 (1963).
- Smith Kline & French Lab., GB 829246 (1960).
- Sherico Ltd., GB 833474 (1960).
- R.C. Merril, H.L. Yale, U.S. Patent 3,194,733 (1965).
- Yale, Harry L.; Sowinski, Francis (1960). "4-{3-[10-(2-Trifluoromethyl)-phenothiazinyl]-propyl}-1-piperazine-ethanol1 and Related Compounds. II2". Journal of the American Chemical Society 82: 2039. doi:10.1021/ja01493a047.
- E.L. Anderson, G.B. Bellizona, P.N. Craig, G.E. Jaffe, K.P. Janewaes, C. Kaiser, B.M. Hester, E.J. Nikawitz, A. Pavloff, H.E. Reift, Ch.L. Zirkle, Arzneim.-Forsch., 12, 937 (1962).
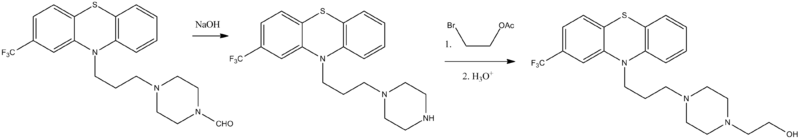 Alkylation of 2-trifluoromethylphenothiazine using 4-formyl-1-piperazineylpropylchloride in the presence of sodamide synthesizes 2-trifluoromethyl-10-[3-(4-formyl-1-piperazinyl)propyl]phenothiazine. Further alkaline hydrolysis removes the N-formyl group, giving 2-trifluoromethyl-10-[3-(1-piperazinyl)propyl]phenothiazine. This is alkylated by 2-bromoethanol-1 acetate, which upon further acidic hydrolysis removes the protecting acetyl group, yielding fluphenazine.
Alkylation of 2-trifluoromethylphenothiazine using 4-formyl-1-piperazineylpropylchloride in the presence of sodamide synthesizes 2-trifluoromethyl-10-[3-(4-formyl-1-piperazinyl)propyl]phenothiazine. Further alkaline hydrolysis removes the N-formyl group, giving 2-trifluoromethyl-10-[3-(1-piperazinyl)propyl]phenothiazine. This is alkylated by 2-bromoethanol-1 acetate, which upon further acidic hydrolysis removes the protecting acetyl group, yielding fluphenazine.- E.L. Anderson, G.B. Bellizona, P.N. Craig, G.E. Jaffe, K.P. Janewaes, C. Kaiser, B.M. Hester, E.J. Nikawitz, A. Pavloff, H.E. Reift, Ch.L. Zirkle, Arzneim.-Forsch., 12, 937 (1962).
- J.W. Cusic, U.S. Patent 2,766,235 (1956).
References
- "Fluphenazine". Davis's Drug Guide for Nurses, Eighth Edition. F.A. Davis Company, 2005. ISBN 0-8036-2455-7.
- FLUPHENAZINE DICHLORHYDRATE [Article in French] Biam Last Updated: 11 December 2000. Accessed 14 September 2005.
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsHistaminergics Receptor
ligandsAgonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorphenamine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; "3rd generation": Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc) • Serotonin antagonist and reuptake inhibitors (Trazodone, Nefazodone) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc) • Atypical antipsychotics (Clozapine, Olanzapine, Quetiapine, etc)Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Burimamide • Cimetidine • Ebrotidine • Famotidine • Lafutidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Niperotidine • Nizatidine • Oxmetidine • Ranitidine • RoxatidineAgonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • Pitolisant • SCH-79,687 • Thioperamide • VUF-5,681Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002Reuptake
inhibitorsVMAT inhibitorsEnzyme
inhibitorsHDC inhibitorsCatechin • Meciadanol • Naringenin • TritoqualineHNMT inhibitorsDAO inhibitorsAminoguanidineOthers L-HistidinePiperazines Simple piperazines
(no additional rings)1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPESPhenylpiperazines Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Diphenylpiperazine • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • Mefway • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635Benzylpiperazines 2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • VesnarinoneDiphenylalkylpiperazines
(benzhydrylalkylpiperazines)Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • VanoxerinePyrimidinylpiperazines Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • ZalospironePyridinylpiperazines Atevirdine • Azaperone • PyridinylpiperazineBenzo(iso)thiazolylpiperazines Tricyclics
(piperazine attached via side chain)Amoxapine • Clopenthixol • Clozapine • Flupentixol • Fluphenazine • Loxapine • Olanzapine • Opipramol • Perazine • Perphenazine • Pirenzepine • Prochlorperazine • Thiethylperazine • Thiothixene • Trifluoperazine • ZuclopenthixolOthers 6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • ZipeprolTricyclics Classes Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • ThioxantheneAntidepressants 7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • TrimipramineAntihistamines Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • ThiazinamiumAntipsychotics Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • ZuclopenthixolOthers Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • ProfenamineCategories:- Alcohols
- Antiemetics
- Organofluorides
- Phenothiazines
- Piperazines
Wikimedia Foundation. 2010.
Look at other dictionaries:
Fluphenazine — Fluphénazine Fluphénazine Général Nom IUPAC 2 [4 [3 [2 (trifluoromethyl) 10H phenothiazin 10 yl]propyl] piperazin 1 yl]ethanol … Wikipédia en Français
Fluphénazine — Général Nom IUPAC 2 [4 [3 [2 (trifluoromethyl) 10H phenothiazin 10 yl]propyl] piperazin 1 yl]ethanol … Wikipédia en Français
fluphenazine — A drug that is used to treat mental and emotional disorders and is being studied in the treatment of multiple myeloma. Fluphenazine may stop tumor growth by keeping myeloma cells from dividing and causing them to die. It is a type of… … English dictionary of cancer terms
fluphenazine hydrochloride — A drug that is used to treat mental and emotional disorders and is being studied in the treatment of multiple myeloma. Fluphenazine hydrochloride may stop tumor growth by keeping myeloma cells from dividing and causing them to die. It is a type… … English dictionary of cancer terms
fluphenazine decanoate — [USP] the decanoate ester of fluphenazine, having the same actions as the hydrochloride salt, but of longer duration; administered subcutaneously or intramuscularly in maintenance therapy for psychotic disorders … Medical dictionary
fluphenazine enanthate — [USP] the enanthate ester of fluphenazine, having the same uses as the hydrochloride salt, but of longer duration; administered intramuscularly and subcutaneously … Medical dictionary
fluphenazine hydrochloride — [USP] the dihydrochloride salt of fluphenazine, used as an antipsychotic; administered orally and intramuscularly … Medical dictionary
fluphenazine — noun Etymology: fluor + phenazine Date: 1959 a tranquilizer C22H26F3N3OS used especially in the form of its hydrochloride … New Collegiate Dictionary
fluphenazine — /flooh fen euh zeen /, n. Pharm. a potent tranquilizer, C22H26F3N3OS, derived from phenothiazine and used in various forms for the management of certain neurological or psychotic disorders and for short term treatment of acute anxiety. [1955 60;… … Universalium
fluphenazine — noun An antipsychotic drug used in the treatment of schizophrenia etc … Wiktionary
