- Homologous recombination
-
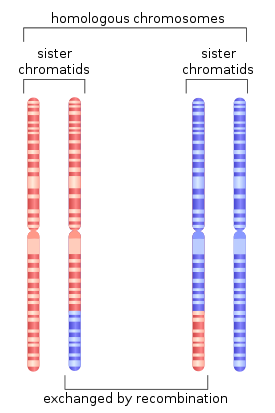 Figure 1. During meiosis, homologous recombination can produce new combinations of genes as shown here between similar but not identical copies of human chromosome 1.
Figure 1. During meiosis, homologous recombination can produce new combinations of genes as shown here between similar but not identical copies of human chromosome 1.
Homologous recombination is a type of genetic recombination in which nucleotide sequences are exchanged between two similar or identical molecules of DNA. It is most widely used by cells to accurately repair harmful breaks that occur on both strands of DNA, known as double-strand breaks. Homologous recombination also produces new combinations of DNA sequences during meiosis, the process by which eukaryotes make gamete cells, like sperm and egg cells in animals. These new combinations of DNA represent genetic variation in offspring, which in turn enables populations to adapt during the course of evolution.[1] Homologous recombination is also used in horizontal gene transfer to exchange genetic material between different strains and species of bacteria and viruses.
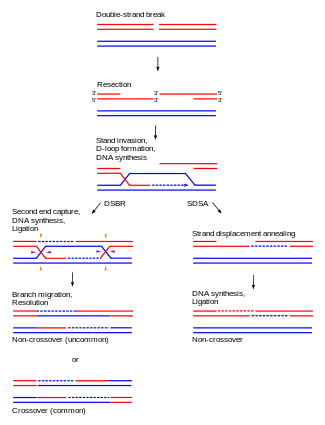
Although homologous recombination varies widely among different organisms and cell types, most forms of it involve the same basic steps. After a double-strand break occurs, sections of DNA around the 5' ends of the break are cut away in a process called resection. In the strand invasion step that follows, an overhanging 3' end of the broken DNA molecule then "invades" a similar or identical DNA molecule that is not broken. After strand invasion, one or two cross-shaped structures called Holliday junctions connect the two DNA molecules. Depending on how the two junctions are cut by enzymes, the type of homologous recombination that occurs in meiosis results in either chromosomal crossover or non-crossover. Homologous recombination that occurs during DNA repair tends to result in non-crossover products, in effect restoring the damaged DNA molecule as it existed before the double-strand break.
Homologous recombination is conserved across all three domains of life as well as viruses, suggesting that it is a nearly universal biological mechanism. The discovery of genes for homologous recombination in protists—a diverse group of eukaryotic microorganisms—has been interpreted as evidence that meiosis emerged early in the evolution of eukaryotes. Since their dysfunction has been strongly associated with increased susceptibility to several types of cancer, the proteins that facilitate homologous recombination are topics of active research. Homologous recombination is also used as a technique in molecular biology for introducing genetic changes into target organisms. The development of gene targeting techniques that rely on homologous recombination was the subject of the 2007 Nobel Prize for Physiology or Medicine.
Contents
History and discovery
 Figure 2. An early illustration of crossing over from Thomas Hunt Morgan
Figure 2. An early illustration of crossing over from Thomas Hunt Morgan
In the early 1900s, William Bateson and Reginald Punnett found an exception to one of the principles of inheritance originally described by Gregor Mendel in the 1860s. In contrast to Mendel's notion that traits are independently assorted when passed from parent to child—for example that a cat's hair color and its tail length are inherited independent of each other—Bateson and Punnett showed that certain genes associated with physical traits can be inherited together, or genetically linked.[2][3] In 1911, after observing that linked traits could on occasion be inherited separately, Thomas Hunt Morgan suggested that "crossovers" can occur between linked genes,[4] where one of the linked genes physically crosses over to a different chromosome. Two decades later, Barbara McClintock and Harriet Creighton demonstrated that chromosomal crossover occurs during meiosis,[5][6] the process of cell division by which sperm and egg cells are made. Within the same year as McClintock's discovery, Curt Stern showed that crossing over—later called "recombination"—could also occur in somatic cells like white blood cells and skin cells that divide through mitosis.[5][7]
In 1947, the microbiologist Joshua Lederberg showed that bacteria—which had been assumed to reproduce only asexually through binary fission—are capable of genetic recombination, which is more similar to sexual reproduction. This work established E. coli as a model organism in genetics,[8] and helped Lederberg win the 1958 Nobel Prize in Physiology or Medicine.[9] Building on studies in fungi, in 1964 Robin Holliday proposed a model for recombination in meiosis which introduced key details of how the process can work, including the exchange of material between chromosomes through Holliday junctions.[10] In 1983, Jack Szostak and colleagues presented a model now known as the DSBR pathway, which accounted for observations not explained by the Holliday model.[10][11] During the next decade, experiments in Drosophila, budding yeast and mammalian cells led to the emergence of other models of homologous recombination, called SDSA pathways, which do not always rely on Holliday junctions.[10]
In eukaryotes
Homologous recombination is essential to cell division in eukaryotes like plants, animals, fungi and protists. In cells that divide through mitosis, homologous recombination repairs double-strand breaks in DNA caused by ionizing radiation or DNA-damaging chemicals.[12] Left unrepaired, these double-strand breaks can cause large-scale rearrangement of chromosomes in somatic cells,[13] which can in turn lead to cancer.[14]
In addition to repairing DNA, homologous recombination also helps produce genetic diversity when cells divide in meiosis to become specialized gamete cells—sperm or egg cells in animals, pollen or ovules in plants, and spores in fungi. It does so by facilitating chromosomal crossover, in which regions of similar but not identical DNA are exchanged between homologous chromosomes.[15][16] This creates new, possibly beneficial combinations of genes, which can give offspring an evolutionary advantage.[17] Chromosomal crossover begins when a protein called Spo11 makes a targeted double-strand break in DNA.[18] The sites of these double-strand breaks often occur at recombination hotspots, regions in chromosomes that are about 1,000–2,000 base pairs in length and have high rates of recombination. The absence of a recombination hotspot between two genes on the same chromosome often means that those genes will be inherited by future generations in equal proportion. This represents linkage between the two genes greater than would be expected from genes that independently assort during meiosis.[19]
Timing within the cell cycle
 Figure 3. Homologous recombination repairs DNA before the cell enters mitosis (M phase). It occurs only during and shortly after DNA replication, during the S and G2 phases of the cell cycle.
Figure 3. Homologous recombination repairs DNA before the cell enters mitosis (M phase). It occurs only during and shortly after DNA replication, during the S and G2 phases of the cell cycle.
Double-strand breaks can be repaired through homologous recombination or through non-homologous end joining (NHEJ). NHEJ is a DNA repair mechanism which, unlike homologous recombination, does not require a long homologous sequence to guide repair. Whether homologous recombination or NHEJ is used to repair double-strand breaks is largely determined by the phase of cell cycle. Homologous recombination repairs DNA before the cell enters mitosis (M phase). It occurs during and shortly after DNA replication, in the S and G2 phases of the cell cycle, when sister chromatids are more easily available.[20] Compared to homologous chromosomes, which are similar to another chromosome but often have different alleles, sister chromatids are an ideal template for homologous recombination because they are an identical copy of a given chromosome. In contrast to homologous recombination, NHEJ is predominant in the G1 phase of the cell cycle, when the cell is growing but not yet ready to divide. It occurs less frequently after the G1 phase, but maintains at least some activity throughout the cell cycle. The mechanisms that regulate homologous recombination and NHEJ throughout the cell cycle vary widely between species.[21]
Cyclin-dependent kinases (CDKs), which modify the activity of other proteins by adding phosphate groups to (that is, phosphorylating) them, are important regulators of homologous recombination in eukaryotes.[21] When DNA replication begins in budding yeast, the cyclin-dependent kinase Cdc28 begins homologous recombination by phosphorylating the Sae2 protein.[22] After being so activated by the addition of a phosphate, Sae2 uses its endonuclease activity to make a clean cut near a double-strand break in DNA. This allows a three-part protein known as the MRX complex to bind to DNA, and begins a series of protein-driven reactions that exchange material between two DNA molecules.[23]
Models
Two primary models for how homologous recombination repairs double-strand breaks in DNA are the DSBR pathway (sometimes called the double Holliday junction model) and the synthesis-dependent strand annealing (SDSA) pathway.[24] The two pathways are similar in their first several steps. After a double-strand break occurs, the MRX complex (MRN complex in humans) binds to DNA on either side of the break. Next a resection, in which DNA around the 5' ends of the break is cut back, is carried out in two distinct steps. In the first step of resection, the MRX complex recruits the Sae2 protein. The two proteins then trim back the 5' ends on either side of the break to create short 3' overhangs of single-strand DNA. In the second step, 5'→3' resection is continued by the Sgs1 helicase and the Exo1 and Dna2 nucleases. As a helicase, Sgs1 "unzips" the double-strand DNA, while Exo1 and Dna2's nuclease activity allows them to cut the single-stranded DNA produced by Sgs1.[22]
The RPA protein, which has high affinity for single-stranded DNA, then binds the 3' overhangs.[25] With the help of several other proteins that mediate the process, the Rad51 protein (and Dmc1, in meiosis) then forms a filament of nucleic acid and protein on the single strand of DNA coated with RPA. This nucleoprotein filament then begins searching for DNA sequences similar to that of the 3' overhang. After finding such a sequence, the single-stranded nucleoprotein filament moves into (invades) the similar or identical recipient DNA duplex in a process called strand invasion. In cells that divide through mitosis, the recipient DNA duplex is generally a sister chromatid, which is identical to the damaged DNA molecule and provides a template for repair. In meiosis, however, the recipient DNA tends to be from a similar but not necessarily identical homologous chromosome.[24] A displacement loop (D-loop) is formed during strand invasion between the invading 3' overhang strand and the homologous chromosome. After strand invasion, a DNA polymerase extends the end of the invading 3' strand by synthesizing new DNA. This changes the D-loop to a cross-shaped structure known as a Holliday junction. Following this, more DNA synthesis occurs on the invading strand (i.e., one of the original 3' overhangs), effectively restoring the strand on the homologous chromosome that was displaced during strand invasion.[24]
DSBR pathway
After the stages of resection, strand invasion and DNA synthesis, the DSBR and SDSA pathways become distinct.[24] The DSBR pathway is unique in that the second 3' overhang (which was not involved in strand invasion) also forms a Holliday junction with the homologous chromosome. The double Holliday junctions are then converted into recombination products by nicking endonucleases, a type of restriction endonuclease which cuts only one DNA strand. The DSBR pathway commonly results in crossover, though it can sometimes result in non-crossover products; the ability of a broken DNA molecule to collect sequences from separated donor loci was shown in mitotic budding yeast using plasmids or endonuclease induction of chromosomal events. [26][27] Because of this tendency for chromosomal crossover, the DSBR pathway is a likely model of how homologous recombination occurs during meiosis.[15]
Whether recombination in the DSBR pathway results in chromosomal crossover is determined by how the double Holliday junction is cut, or "resolved". Chromosomal crossover will occur if one Holliday junction is cut on the crossing strand and the other Holliday junction is cut on the non-crossing strand (in Figure 4, along the horizontal purple arrowheads at one Holliday junction and along the vertical orange arrowheads at the other). Alternatively, if the two Holliday junctions are cut on the crossing strands (along the horizontal purple arrowheads at both Holliday junctions in Figure 4), then chromosomes without crossover will be produced.[28]
SDSA pathway
Homologous recombination via the SDSA pathway occurs in cells that divide through mitosis and results in non-crossover products. In this model, the invading 3' strand is extended along the recipient DNA duplex by a DNA polymerase, and is released as the Holliday junction between the donor and recipient DNA molecules slides in a process called branch migration. The newly synthesized 3' end of the invading strand is then able to anneal to the other 3' overhang in the damaged chromosome through complementary base pairing. After the strands anneal, a small flap of DNA can sometimes remain. Any such flaps are removed, and the SDSA pathway finishes with the resealing, also known as ligation, of any remaining single-stranded gaps.[29]
SSA pathway
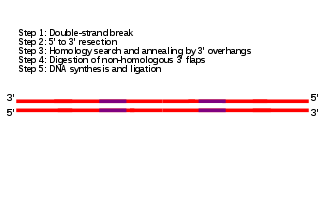
The single-strand annealing (SSA) pathway of homologous recombination repairs double-strand breaks between two repeat sequences. The SSA pathway is unique in that it does not require a separate similar or identical molecule of DNA, like the DSBR or SDSA pathways of homologous recombination. Instead, the SSA pathway only requires a single DNA duplex, and uses the repeat sequences as the identical sequences that homologous recombination needs for repair. The pathway is relatively simple in concept: after two strands of the same DNA duplex are cut back around the site of the double-strand break, the two resulting 3' overhangs then align and anneal to each other, restoring the DNA as a continuous duplex.[29][30]
As DNA around the double-strand break is cut back, the single-stranded 3' overhangs being produced are coated with the RPA protein, which prevents the 3' overhangs from sticking to themselves.[31] A protein called Rad52 then binds each of the repeat sequences on either side of the break, and aligns them to enable the two complementary repeat sequences to anneal.[31] After annealing is complete, leftover non-homologous flaps of the 3' overhangs are cut away by a set of nucleases, known as Rad1/Rad10, which are brought to the flaps by the Saw1 and Slx4 proteins.[31][32] New DNA synthesis fills in any gaps, and ligation restores the DNA duplex as two continuous strands.[33] The DNA sequence between the repeats is always lost, as is one of the two repeats. The SSA pathway is considered mutagenic since it results in such deletions of genetic material.[29]
BIR pathway
During DNA replication, double-strand breaks can sometimes be encountered at replication forks as DNA helicase unzips the template strand. These defects are repaired in the break-induced replication (BIR) pathway of homologous recombination. The precise molecular mechanisms of the BIR pathway remain unclear. Three proposed mechanisms have strand invasion as an initial step, but differ in how they model the migration of the D-loop and later phases of recombination.[34]
The BIR pathway can also help to maintain the length of telomeres, regions of DNA at the end of eukaryotic chromosomes, in the absence of (or in cooperation with) telomerase. Without working copies of the telomerase enzyme, telomeres typically shorten with each cycle of mitosis, which eventually blocks cell division and leads to senescence. In budding yeast cells where telomerase have been inactivated through mutations, two types of "survivor" cells have been observed to avoid senescence longer than expected by elongating their telomeres through BIR pathways.[34]
Maintaining telomere length is critical for cell immortalization, a key feature of cancer. Most cancers maintain telomeres by upregulating telomerase. However, in several types of human cancer, a BIR-like pathway helps to sustain some tumors by acting as an alternative mechanism of telomere maintenance.[35] This fact has lead scientists to investigate whether such recombination-based mechanisms of telomere maintenance could thwart anti-cancer drugs like telomerase inhibitors.[36]
In bacteria
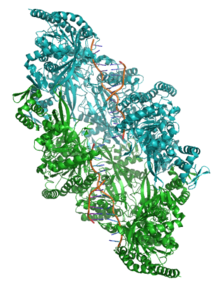 Figure 6. Crystal structure of two chains of the RecA protein bound to DNA.[37] A double-strand break and two adjacent 3' overhangs are visible.
Figure 6. Crystal structure of two chains of the RecA protein bound to DNA.[37] A double-strand break and two adjacent 3' overhangs are visible.
Homologous recombination is a major DNA repair process in bacteria. It is also important for producing genetic diversity in bacterial populations, although the process differs substantially from meiotic recombination, which brings about diversity in eukaryotic genomes. Homologous recombination has been most studied and is best understood for Escherichia coli.[38] Double-strand DNA breaks in bacteria are repaired by the RecBCD pathway of homologous recombination. Breaks that occur on only one of the two DNA strands, known as single-strand gaps, are thought to be repaired by the RecF pathway.[39] Both the RecBCD and RecF pathways include a series of reactions known as branch migration, in which single DNA strands are exchanged between two intercrossed molecules of duplex DNA, and resolution, in which those two intercrossed molecules of DNA are cut apart and restored to their normal double-stranded state.
RecBCD pathway
 Figure 7A. Molecular model for the RecBCD pathway of recombination. This model is based on reactions of DNA and RecBCD with ATP in excess over Mg2+ ions. Step a: RecBCD binds to a double-stranded DNA end. Step b: RecBCD unwinds DNA. RecD is a fast helicase on the 5’-ended strand, and RecB is a slower helicase on the 3’-ended strand (that with an arrowhead).[40] This produces two single-stranded (ss) DNA tails and one ss loop. The loop and tails enlarge as RecBCD moves along the DNA. Step c: The two tails anneal to produce a second ss DNA loop, and both loops move and grow. Step d: Upon reaching the Chi hotspot sequence RecBCD nicks the 3’-ended strand. Further unwinding produces a long 3’-ended ss tail with Chi near its end. Step e: RecBCD loads RecA protein onto the Chi tail. At some undetermined point, the RecBCD subunits disassemble. Step f: The RecA-ssDNA complex invades an intact homologous duplex DNA to produce a D-loop, which can be resolved into intact, recombinant DNA in two ways. Step g: The D-loop is cut and anneals with the gap in the first DNA to produce a Holliday junction. Resolution of the Holliday junction (cutting, swapping of strands, and ligation) at the open arrowheads by some combination of RuvABC and RecG produces two recombinants of reciprocal type. Step h: The 3’ end of the Chi tail primes DNA synthesis, from which a replication fork can be generated. Resolution of the fork at the open arrowheads produces one recombinant (non-reciprocal) DNA, one parental-type DNA, and one DNA fragment.
Figure 7A. Molecular model for the RecBCD pathway of recombination. This model is based on reactions of DNA and RecBCD with ATP in excess over Mg2+ ions. Step a: RecBCD binds to a double-stranded DNA end. Step b: RecBCD unwinds DNA. RecD is a fast helicase on the 5’-ended strand, and RecB is a slower helicase on the 3’-ended strand (that with an arrowhead).[40] This produces two single-stranded (ss) DNA tails and one ss loop. The loop and tails enlarge as RecBCD moves along the DNA. Step c: The two tails anneal to produce a second ss DNA loop, and both loops move and grow. Step d: Upon reaching the Chi hotspot sequence RecBCD nicks the 3’-ended strand. Further unwinding produces a long 3’-ended ss tail with Chi near its end. Step e: RecBCD loads RecA protein onto the Chi tail. At some undetermined point, the RecBCD subunits disassemble. Step f: The RecA-ssDNA complex invades an intact homologous duplex DNA to produce a D-loop, which can be resolved into intact, recombinant DNA in two ways. Step g: The D-loop is cut and anneals with the gap in the first DNA to produce a Holliday junction. Resolution of the Holliday junction (cutting, swapping of strands, and ligation) at the open arrowheads by some combination of RuvABC and RecG produces two recombinants of reciprocal type. Step h: The 3’ end of the Chi tail primes DNA synthesis, from which a replication fork can be generated. Resolution of the fork at the open arrowheads produces one recombinant (non-reciprocal) DNA, one parental-type DNA, and one DNA fragment.
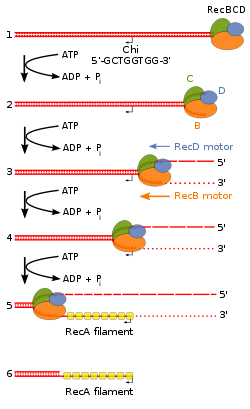 Figure 7B. Beginning of the RecBCD pathway. This model is based on reactions of DNA and RecBCD with Mg2+ ions in excess over ATP. Step 1: RecBCD binds to a DNA double strand break. Step 2: RecBCD initiates unwinding of the DNA duplex through ATP-dependent helicase activity. Step 3: RecBCD continues its unwinding and moves down the DNA duplex, cleaving the 3' strand much more frequently than the 5' strand. Step 4: RecBCD encounters a Chi sequence and stops digesting the 3' strand; cleavage of the 5' strand is significantly increased. Step 5: RecBCD loads RecA onto the 3' strand. Step 6: RecBCD unbinds from the DNA duplex, leaving a RecA nucleoprotein filament on the 3' tail.[41]
Figure 7B. Beginning of the RecBCD pathway. This model is based on reactions of DNA and RecBCD with Mg2+ ions in excess over ATP. Step 1: RecBCD binds to a DNA double strand break. Step 2: RecBCD initiates unwinding of the DNA duplex through ATP-dependent helicase activity. Step 3: RecBCD continues its unwinding and moves down the DNA duplex, cleaving the 3' strand much more frequently than the 5' strand. Step 4: RecBCD encounters a Chi sequence and stops digesting the 3' strand; cleavage of the 5' strand is significantly increased. Step 5: RecBCD loads RecA onto the 3' strand. Step 6: RecBCD unbinds from the DNA duplex, leaving a RecA nucleoprotein filament on the 3' tail.[41]
The RecBCD pathway is the main recombination pathway used in bacteria to repair double-strand breaks in DNA.[42] These double-strand breaks can be caused by UV light and other radiation, as well as chemical mutagens. Double-strand breaks may also arise by DNA replication through a single-strand nick or gap. Such a situation causes what is known as a collapsed replication fork and is fixed by several pathways of homologous recombination including the RecBCD pathway.[43]
In this pathway, a three-subunit enzyme complex called RecBCD initiates recombination by binding to a blunt or nearly blunt end of a break in double-strand DNA. After RecBCD binds the DNA end, the RecB and RecD subunits begin unzipping the DNA duplex through helicase activity. The RecB subunit also has a nuclease domain, which cuts the single strand of DNA that emerges from the unzipping process. This unzipping continues until RecBCD encounters a specific nucleotide sequence (5'-GCTGGTGG-3') known as a Chi site.[42]
Upon encountering a Chi site, the activity of the RecBCD enzyme changes drastically.[44][45] DNA unwinding pauses for a few seconds and then resumes at roughly half the initial speed. This is likely because the slower RecB helicase unwinds the DNA after Chi, rather than the faster RecD helicase, which unwinds the DNA before Chi.[40][46] Recognition of the Chi site also changes the RecBCD enzyme so that it cuts the DNA strand with Chi and begins loading multiple RecA proteins onto the single-stranded DNA with the newly generated 3' end. The resulting RecA-coated nucleoprotein filament then searches out similar sequences of DNA on a homologous chromosome. The search process induces stretching of the DNA duplex, which enhances homology recognition (a mechanism termed conformational proofreading [47] [48]). Upon finding such a sequence, the single-stranded nucleoprotein filament moves into the homologous recipient DNA duplex in a process called strand invasion.[49] The invading 3' overhang causes one of the strands of the recipient DNA duplex to be displaced, to form a D-loop. If the D-loop is cut, another swapping of strands forms a cross-shaped structure called a Holliday junction.[42] Resolution of the Holliday junction by some combination of RuvABC or RecG can produce two recombinant DNA molecules with reciprocal genetic types, if the two interacting DNA molecules differ genetically. Alternatively, the invading 3’ end near Chi can prime DNA synthesis and form a replication fork. This type of resolution produces only one type of recombinant (non-reciprocal).
RecF pathway
Further information: RecF pathwayBacteria appear to use the RecF pathway of homologous recombination to repair single-strand gaps in DNA. When the RecBCD pathway is inactivated by mutations and additional mutations inactivate the SbcCD and ExoI nucleases, the RecF pathway can also repair DNA double-strand breaks.[50] In the RecF pathway the RecQ helicase unwinds the DNA and the RecJ nuclease degrades the strand with a 5’ end, leaving the strand with the 3’ end intact. RecA protein binds to this strand and is either aided by the RecF, RecO, and RecR proteins or stabilized by them. The RecA nucleoprotein filament then searches for a homologous DNA and exchanges places with the identical or nearly identical strand in the homologous DNA.
Although the proteins and specific mechanisms involved in their initial phases differ, the two pathways are similar in that they both require single-stranded DNA with a 3’ end and the RecA protein for strand invasion. The pathways are also similar in their phases of branch migration, in which the Holliday junction slides in one direction, and resolution, in which the Holliday junctions are cleaved apart by enzymes.[51][52] The alternative, non-reciprocal type of resolution may also occur by either pathway.
Branch migration
Immediately after strand invasion, the Holliday junction moves along the linked DNA during the branch migration process. It is in this movement of the Holliday junction that base pairs between the two homologous DNA duplexes are exchanged. To catalyze branch migration, the RuvA protein first recognizes and binds to the Holliday junction and recruits the RuvB protein to form the RuvAB complex. Two sets of the RuvB protein, which each form a ring-shaped ATPase, are loaded onto opposite sides of the Holliday junction, where they act as twin pumps that provide the force for branch migration. Between those two rings of RuvB, two sets of the RuvA protein assemble in the center of the Holliday junction such that the DNA at the junction is sandwiched between each set of RuvA. The strands of both DNA duplexes—the "donor" and the "recipient" duplexes—are unwound on the surface of RuvA as they are guided by the protein from one duplex to the other.[53][54]
Resolution
In the resolution phase of recombination, any Holliday junctions formed by the strand invasion process are cut, thereby restoring two separate DNA molecules. This cleavage is done by RuvAB complex interacting with RuvC, which together form the RuvABC complex. RuvC is an endonuclease that cuts the degenerate sequence 5'-(A/T)TT(G/C)-3'. The sequence is found frequently in DNA, about once every 64 nucleotides.[54] Before cutting, RuvC likely gains access to the Holliday junction by displacing one of the two RuvA tetramers covering the DNA there.[53] Recombination results in either "splice" or "patch" products, depending on how RuvC cleaves the Holliday junction.[54] Splice products are crossover products, in which there is a rearrangement of genetic material around the site of recombination. Patch products, on the other hand, are non-crossover products in which there is no such rearrangement and there is only a "patch" of hybrid DNA in the recombination product.[55]
Facilitating genetic transfer
Homologous recombination is an important method of integrating donor DNA into a recipient organism's genome in horizontal gene transfer, the process by which an organism incorporates foreign DNA from another organism without being the offspring of that organism. Homologous recombination requires incoming DNA to be highly similar to the recipient genome, and so horizontal gene transfer is usually limited to similar bacteria.[56] Studies in several species of bacteria have established that there is a log-linear decrease in recombination frequency with increasing difference in sequence between host and recipient DNA.[57][58][59]
In bacterial conjugation, where DNA is transferred between bacteria through direct cell-to-cell contact, homologous recombination helps integrate foreign DNA into the host genome via the RecBCD pathway. The RecBCD enzyme promotes recombination after DNA is converted from single-strand DNA–in which form it originally enters the bacterium–to double-strand DNA during replication. The RecBCD pathway is also essential for the final phase of transduction, a type of horizontal gene transfer in which DNA is transferred from one bacterium to another by a virus. Foreign, bacterial DNA is sometimes misincorporated in the capsid head of bacteriophage virus particles as DNA is packaged into new bacteriophages during viral replication. When these new bacteriophages infect other bacteria, DNA from the previous host bacterium is injected into the new bacterial host as double-strand DNA. The RecBCD enzyme then incorporates this double-strand DNA into the genome of the new bacterial host.[42]
In viruses
Homologous recombination occurs in several groups of viruses. In DNA viruses such as herpesvirus, recombination occurs through a break-and-rejoin mechanism like in bacteria and eukaryotes.[60] There is also evidence for recombination in some RNA viruses, specifically positive-sense ssRNA viruses like retroviruses, picornaviruses, and coronaviruses. There is controversy over whether homologous recombination occurs in negative-sense ssRNA viruses like influenza.[61]
In RNA viruses, homologous recombination can be either precise or imprecise. In the precise type of RNA-RNA recombination, there is no difference between the two parental RNA sequences and the resulting crossover RNA region. Because of this, it is often difficult to determine the location of crossover events between two recombining RNA sequences. In imprecise RNA homologous recombination, the crossover region has some difference with the parental RNA sequences – caused by either addition, deletion, or other modification of nucleotides. The level of precision in crossover is controlled by the sequence context of the two recombining strands of RNA: sequences rich in adenine and uracil decrease crossover precision.[62]
Homologous recombination is important in facilitating viral evolution.[62][63] For example, if the genomes of two viruses with different disadvantageous mutations undergo recombination, then they may be able to regenerate a fully functional genome. Alternatively, if two similar viruses have infected the same host cell, homologous recombination can allow those two viruses to swap genes and thereby evolve more potent variations of themselves.[63]
Effects of dysfunction
Without proper homologous recombination, chromosomes often incorrectly align for the first phase of cell division in meiosis. This causes chromosomes to fail to properly segregate in a process called nondisjunction. In turn, nondisjunction can cause sperm and ova to have too few or too many chromosomes. Down's syndrome, which is caused by an extra copy of chromosome 21, is one of many abnormalities that result from such a failure of homologous recombination in meiosis.[54][64]
Deficiencies in homologous recombination have been strongly linked to cancer formation in humans. For example, each of the cancer-related diseases Bloom's syndrome, Werner's syndrome and Rothmund-Thomson syndrome are caused by malfunctioning copies of RecQ helicase genes involved in the regulation of homologous recombination: BLM, WRN and RECQ4, respectively.[65] In the cells of Bloom's syndrome patients, who lack a working copy of the BLM protein, there is an elevated rate of homologous recombination.[66] Experiments in mice deficient in BLM have suggested that the mutation gives rise to cancer through a loss of heterozygosity caused by increased homologous recombination.[67] A loss in heterozygosity refers to the loss of one of two versions—or alleles—of a gene. If one of the lost alleles helps to suppress tumors, like the gene for the retinoblastoma protein for example, then the loss of heterozygosity can lead to cancer.[68]:1236
Decreased rates of homologous recombination cause inefficient DNA repair,[68]:310 which can also lead to cancer.[69] This is the case with BRCA1 and BRCA2, two similar tumor suppressor genes whose malfunctioning has been linked with considerably increased risk for breast and ovarian cancer. Cells missing BRCA1 and BRCA2 have a decreased rate of homologous recombination and increased sensitivity to ionizing radiation, suggesting that decreased homologous recombination leads to increased susceptibility to cancer.[69] Because the only known function of BRCA2 is to help initiate homologous recombination, researchers have speculated that more detailed knowledge of BRCA2's role in homologous recombination may be the key to understanding the causes of breast and ovarian cancer.[69]
Evolutionary origins
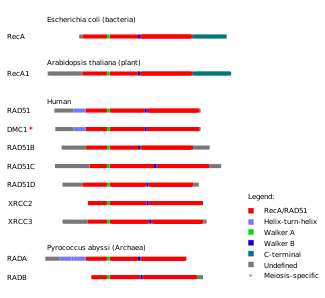 Figure 8. Protein domains in homologous recombination-related proteins are conserved across the three main groups of life: archaea, bacteria and eukaryotes.
Figure 8. Protein domains in homologous recombination-related proteins are conserved across the three main groups of life: archaea, bacteria and eukaryotes.
Based on the similarity of their amino acid sequences, sets of proteins involved in homologous recombination are thought to share common evolutionary origins.[70] One such set of proteins is the RecA/Rad51 protein family, which includes the RecA protein from bacteria, the Rad51 and Dmc1 proteins from eukaryotes and the RadA and RadB proteins from archaea. These proteins play key roles in the beginning stages of homologous recombination in the organisms that express them. The proteins in the RecA/Rad51 protein family share a long conserved region known as the RecA/Rad51 domain. Within this protein domain are two sequence motifs, Walker A motif and Walker B motif. The Walker A and B motifs allow members of the RecA/Rad51 protein family to engage in ATP hydrolysis,[70] which provides energy for the proteins to drive reactions in homologous recombination.[71]
Studies modeling the evolutionary relationships between the Rad51, Dmc1 and RadA proteins indicate that they are monophyletic, or that they share a common molecular ancestor.[70] Within this protein family, Rad51 and Dmc1 are grouped together in a separate clade from RadA. One of the reasons for grouping these three proteins together is that they all possess a modified helix-turn-helix motif, which helps the proteins bind to DNA, toward their N-terminal ends.[70] An ancient gene duplication event of a eukaryotic RecA gene and subsequent mutation has been proposed as a likely origin of the modern RAD51 and DMC1 genes.[70]
The discovery of Dmc1 in several species of Giardia, one of the earliest protists to diverge as a eukaryote, suggests that meiotic homologous recombination—and thus meiosis itself—emerged very early in eukaryotic evolution.[72] In addition to research on Dmc1, studies on the Spo11 protein have provided information on the origins of meiotic recombination.[73] Spo11 is a type II topoisomerase that initiates homologous recombination in meiosis by making targeted double-strand breaks in DNA.[18] Phylogenetic trees based on the sequence of genes similar to SPO11 in animals, fungi, plants, protists and archaea have led scientists to believe that the version Spo11 currently in eukaryotes emerged in the last common ancestor of eukaryotes and archaea.[73]
Technological applications
Gene targeting
 Figure 9. As a developing embryo, this chimeric mouse had the agouti coat color gene introduced into its DNA via gene targeting. Its offspring are homozygous for the agouti gene.
Figure 9. As a developing embryo, this chimeric mouse had the agouti coat color gene introduced into its DNA via gene targeting. Its offspring are homozygous for the agouti gene. Main article: Gene targeting
Main article: Gene targetingMany methods for introducing DNA sequences into organisms to create recombinant DNA and genetically modified organisms use the process of homologous recombination.[74] Also called gene targeting, the method is especially common in yeast and mouse genetics. The gene targeting method in knockout mice uses mouse embryonic stem cells to deliver artificial genetic material (mostly of therapeutic interest), which represses the target gene of the mouse by the principle of homologous recombination. The mouse thereby acts as a working model to understand the effects of a specific mammalian gene. In recognition of their discovery of how homologous recombination can be used to introduce genetic modifications in mice through embryonic stem cells, Mario Capecchi, Martin Evans and Oliver Smithies were awarded the 2007 Nobel Prize for Physiology or Medicine.[75]
Advances in gene targeting technologies which hijack the homologous recombination mechanics of cells are now leading to the development of a new wave of more accurate, isogenic human disease models. These engineered human cell models are thought to more accurately reflect the genetics of human diseases than their mouse model predecessors. This is largely because mutations of interest are introduced into endogenous genes, just as they occur in the real patients, and because they are based on human genomes rather than rat genomes. Furthermore, certain technologies enable the knock-in of a particular mutation rather than just knock-outs associated with older gene targeting technologies.
Protein engineering
Protein engineering with homologous recombination develops chimeric proteins by swapping fragments between two parental proteins. These techniques exploit the fact that recombination can introduce a high degree of sequence diversity while preserving a protein's ability to fold into its tertiary structure, or three-dimensional shape.[76] This stands in contrast to other protein engineering techniques, like random point mutagenesis, in which the probability of maintaining protein function declines exponentially with increasing amino acid substitutions.[77] The chimeras produced by recombination techniques are able to maintain their ability to fold because their swapped parental fragments are structurally and evolutionarily conserved. These recombinable "building blocks" preserve structurally important interactions like points of physical contact between different amino acids in the protein's structure. Computational methods like SCHEMA and statistical coupling analysis can be used to identify structural subunits suitable for recombination.[78][79][80]
Techniques that rely on homologous recombination have been used to engineer new proteins.[78] In a study published in 2007, researchers were able to create chimeras of two enzymes involved in the biosynthesis of isoprenoids, a diverse class of compounds including hormones, visual pigments and certain pheromones. The chimeric proteins acquired an ability to catalyze an essential reaction in isoprenoid biosynthesis—one of the most diverse pathways of biosynthesis found in nature—that was absent in the parent proteins.[81] Protein engineering through recombination has also produced chimeric enzymes with new function in members of a group of proteins known as the cytochrome P450 family,[82] which in humans is involved in detoxifying foreign compounds like drugs, food additives and preservatives.[15]
Cancer therapy
In 2009, researchers reported trial results of a cancer therapy that exploits deficiencies in homologous recombination in certain types of cancer.[83][84] The drug, named olaparib, a PARP1 inhibitor, was shown to shrink or stop the growth of tumors from breast, ovarian and prostate cancers caused by mutations in the BRCA1 or BRCA2 genes. BRCA1 and BRCA2 are necessary for DNA repair by homologous recombination. When BRCA1 or BRCA2 are absent, another type of DNA repair mechanism called base-excision repair usually compensates for the lack of DNA repair by homologous recombination. However, when the PARP1 protein – which is necessary for base-excision repair – is inhibited, DNA repair is drastically reduced, and the cell dies.[83]
By stopping DNA repair in such a fashion, olaparib applies the concept of synthetic lethality to specifically target cancer cells. An article in the New England Journal of Medicine noted that exploiting synthetic lethality was a new direction in anti-cancer drug development.[83] While PARP1 inhibitors represent a novel approach to cancer therapy, researchers have cautioned that they may prove insufficient for treating late-stage, metastatic cancers.[83] Cancer cells can become resistant to a PARP1 inhibitor if they experience deletions of mutations in BRCA2. This undermines the drug's synthetic lethality by restoring cancer cells' ability to repair DNA by homologous recombination.[85]
References
- ^ Alberts, B et al (2002). "Chapter 5: DNA Replication, Repair, and Recombination". Molecular Biology of the Cell (4th ed.). New York: Garland Science. p. 845. ISBN 0-8153-3218-1. OCLC 48122761 57023651 69932405 145080076 48122761 57023651 69932405. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?&rid=mboc4.section.845.
- ^ Bateson, P (August 2002). "William Bateson: a biologist ahead of his time". Journal of Genetics 81 (2): 49–58. doi:10.1007/BF02715900. PMID 12532036. http://www.ias.ac.in/jgenet/Vol81No2/49.pdf.
- ^ "Reginald Crundall Punnett". NAHSTE, University of Edinburgh. http://www.dnaftb.org/dnaftb/concept_5/con5bio.html. Retrieved 3 July 2010.
- ^ Lobo, I; Shaw, K (2008). "Thomas Hunt Morgan, genetic recombination, and gene mapping". Nature Education 1 (1). http://www.nature.com/scitable/topicpage/thomas-hunt-morgan-genetic-recombination-and-gene-496.
- ^ a b Coe, E; Kass, LB (10 May 2005). "Proof of physical exchange of genes on the chromosomes". PNAS 102 (19): 6641–6646. doi:10.1073/pnas.0407340102. PMC 1100733. PMID 15867161. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1100733.
- ^ Creighton, HB; Barbara, B (August 1931). "A correlation of cytological and genetical crossing-over in Zea Mays". PNAS 17 (8): 492–497. doi:10.1073/pnas.17.8.492. PMC 1076098. PMID 16587654. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1076098.
- ^ Stern, C (1931). "Zytologisch-genetische untersuchungen alsbeweise fur die Morgansche theorie des faktoraustauschs". Biol. Zentbl. 51: 547–587.
- ^ Fee, E et al. "The development of bacterial genetics". US National Library of Medicine. http://profiles.nlm.nih.gov/BB/Views/Exhibit/narrative/bacgen1.html. Retrieved 3 July 2010.
- ^ "The Nobel Prize in Physiology or Medicine 1958". Nobelprize.org. http://nobelprize.org/nobel_prizes/medicine/laureates/1958/. Retrieved 3 July 2010.
- ^ a b c Haber, JE; Ira, G; Malkova, A; Sugaware, N (29 Jan 2004). "Repairing a double-strand chromosome break by homologous recombination: revisiting Robin Holliday's model". Philos Trans R Soc Lond B Biol Sci 359 (1441): 79–86. doi:10.1098/rstb.2003.1367. PMC 1693306. PMID 15065659. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1693306.
- ^ Szostak, JW; Orr-Weaver, FW; Rothstein, RJ; Stahl, FW (May 1983). "The double-strand-break repair model for recombination". Cell 33 (1): 25–35. doi:10.1016/0092-8674(83)90331-8. PMID 6380756.
- ^ Lodish H et al. (2000). "12.5: Recombination between Homologous DNA Sites: Double-Strand Breaks in DNA Initiate Recombination". Molecular Cell Biology (4th ed.). W. H. Freeman and Company. ISBN 0-7167-3136-3.
- ^ Griffiths, AJF et al. (1999). "8: Chromosome Mutations: Chromosomal Rearrangements". Modern Genetic Analysis. W. H. Freeman and Company. ISBN 0-7167-3118-5. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mga.section.1212.
- ^ Kumma, KK; Jackson, SP (2001). "DNA double-strand breaks: signaling, repair and the cancer connection". Nature Genetics 27 (3): 247–254. doi:10.1038/85798. PMID 11242102.
- ^ a b c Nelson, DL; Cox, MM (2005). Principles of Biochemistry (4th ed.). Freeman. pp. 980–981. ISBN 978-0716743392.
- ^ Marcon, E; Moens, PB (August 2005). "The evolution of meiosis: recruitment and modification of somatic DNA-repair proteins". Bioessays 27 (8): 795–808. doi:10.1002/bies.20264. PMID 16015600.
- ^ Alberts B et al. (2008). Molecular Biology of the Cell (5th ed.). Garland Science. p. 305. ISBN 978-0-8153-4105-5.
- ^ a b Keeney, S; Giroux, CN; Kleckner, N (7 February 1997). "Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family". Cell 88 (3): 375–384. doi:10.1016/S0092-8674(00)81876-0. PMID 9039264.
- ^ "A DNA recombination "hotspot" in humans is missing in chimps". PLoS Biology 2 (6): e192. June 2004. doi:10.1371/journal.pbio.0020192. PMC 423159. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=423159.
- ^ Alberts B et al. (2008). Molecular Biology of the Cell (5th ed.). Garland Science. p. 303. ISBN 978-0-8153-4105-5.
- ^ a b Shrivastav, M; De Haro, LP; Nickoloff, JA (January 2008). "Regulation of DNA double-strand break repair pathway choice". Cell Research 18 (1): 134–147. doi:10.1038/cr.2007.111. PMID 18157161. http://www.nature.com/cr/journal/v18/n1/full/cr2007111a.html.
- ^ a b Mimitou, EP; Symington, LS (May 2009). "Nucleases and helicases take center stage in homologous recombination". Trends in Biochemical Science 34 (5): 264–272. doi:10.1016/j.tibs.2009.01.010. PMID 19375328.
- ^ Huertas, P; Cortés-Ledesma, F; Sartori, AA; Aguilera, A; Jackson, SP (2 October 2008). "CDK targets Sae2 to control DNA-end resection and homologous recombination". Nature 455 (7213): 689–692. doi:10.1038/nature07215. PMC 2635538. PMID 18716619. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2635538.
- ^ a b c d Sung, P; Klein, H (October 2006). "Mechanism of homologous recombination: mediators and helicases take on regulatory functions". Nature Reviews Molecular Cell Biology 7 (10): 739–750. doi:10.1038/nrm2008. PMID 16926856.
- ^ Wold, MS (1997). "Replication protein A: heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism". Annual Review of Biochemistry 66: 61–92. doi:10.1146/annurev.biochem.66.1.61. PMID 9242902.
- ^ McMahill, MS; Sham, CW; Bishop, DK (November 2007). "Synthesis-dependent strand annealing in meiosis". PLoS Biology 5 (11): e299. doi:10.1371/journal.pbio.0050299. PMC 2062477. PMID 17988174. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2062477.
- ^ Bärtsch, S; Kang, L; Symington, LS (February 2000). "RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates". Mol Cell Biol 20 (4): 1194-1205. PMC 85244. PMID 10648605. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=85244.
- ^ Alberts B et al. (2008). Molecular Biology of the Cell (5th ed.). Garland Science. pp. 312–313. ISBN 978-0-8153-4105-5. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?&rid=mboc4.section.845.
- ^ a b c Helleday, T; Lo, J; Van Gent, DC; Engelward, BP (1 July 2007). "DNA double-strand break repair: from mechanistic understanding to cancer treatment". DNA Repair (Amst.) 6 (7): 923–935. doi:10.1016/j.dnarep.2007.02.006. PMID 17363343.
- ^ Haber lab. "Single-strand annealing". "Brandeis University". http://www.bio.brandeis.edu/haberlab/jehsite/ssa.html. Retrieved 3 July 2010.
- ^ a b c Lyndaker, AM; Alani, E (March 2009). "A tale of tails: insights into the coordination of 3 end processing during homologous recombination". Bioessays 31 (3): 315–321. doi:10.1002/bies.200800195. PMC 2958051. PMID 19260026. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2958051.
- ^ Mimitou, EP; Symington, LS (September 2009). "DNA end resection: Many nucleases make light work". DNA Repair 8 (9): 983–995. doi:10.1016/j.dnarep.2009.04.017. PMC 2760233. PMID 19473888. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2760233.
- ^ Pâques, F; Haber, JE (June 1999). "Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae". Microbiology and molecular biology reviews : MMBR 63 (2): 349–404. PMC 98970. PMID 10357855. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=98970.
- ^ a b McEachern, MJ; Haber, JE (2006). "Break-induced replication and recombinational telomere elongation in yeast". Annual Review of Biochemistry 75: 111–135. doi:10.1146/annurev.biochem.74.082803.133234. PMID 16756487.
- ^ Morrish, TA; Greider, CW; Haber, James E. (January 2009). Haber, James E.. ed. "Short telomeres initiate telomere recombination in primary and tumor cells". PLoS Genetics 5 (1): e1000357. doi:10.1371/journal.pgen.1000357. PMC 2627939. PMID 19180191. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2627939.
- ^ Muntoni, A; Reddel, RR (October 2005). "The first molecular details of ALT in human tumor cells". Human Molecular Genetics 14 (Review Issue 2): R191–R196. doi:10.1093/hmg/ddi266. PMID 16244317. http://hmg.oxfordjournals.org/cgi/content/full/14/suppl_2/R191.
- ^ PDB 3cmt; Chen Z, Yang H, Pavletich NP (May 2008). "Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures". Nature 453 (7194): 489–4. doi:10.1038/nature06971. PMID 18497818.
- ^ Kowalczykowski, SC; Dixon, DA; Eggleston, AK; Lauder, SD; Rehrauer, WM (September 1994). "Biochemistry of homologous recombination in Escherichia coli". Microbiology and Molecular Biology Reviews 58 (3): 401–465. PMC 372975. PMID 7968921. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=372975.
- ^ Rocha, EPC; Cornet, E; Michel, B (August 2005). "Comparative and evolutionary analysis of the bacterial homologous recombination systems". PLoS Genetics 1 (2): e15. doi:10.1371/journal.pgen.0010015. PMC 1193525. PMID 16132081. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1193525.
- ^ a b Taylor, AF; Smith, GR (June 2003). "RecBCD enzyme is a DNA helicase with fast and slow motors of opposite polarity". Nature 423 (6942): 889–93. doi:10.1038/nature01674. PMID 12815437.
- ^ Singleton, MR; Dillingham, MS; Gaudier, M; Kowalczykowski, SC; Wigley, DB (11 November 2004). "Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks". Nature 432 (7014): 187–193. doi:10.1038/nature02988. PMID 15538360. http://www2.biochemtech.uni-halle.de/xray/seminars/8_3.pdf.
- ^ a b c d Dillingham, MS; Kowalczykowski, SC (December 2008). "RecBCD enzyme and the repair of double-stranded DNA breaks". Microbiology and Molecular Biology Reviews 72 (4): 642–671. doi:10.1128/MMBR.00020-08. PMC 2593567. PMID 19052323. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2593567.
- ^ Michel, B; Boubakri, H; Baharoglu, Z; LeMasson, M; M, R; Lestini, R (1 July 2007). "Recombination proteins and rescue of arrested replication forks". DNA Repair 6 (7): 967–980. doi:10.1016/j.dnarep.2007.02.016. PMID 17395553.
- ^ Amundsen, SK; Taylor, AF; Reddy, M; Smith, G. R.; Smith, GR (December 2007). "Intersubunit signaling in RecBCD enzyme, a complex protein machine regulated by Chi hot spots". Genes Dev 21 (24): 3296–307. doi:10.1101/gad.1605807. PMC 2113030. PMID 18079176. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2113030.
- ^ Spies, M; Bianco, PR; Dillingham, MS; Handa, N; Baskin, RJ; Kowalczykowski, SC (5 September 2003). "A molecular throttle: the recombination hotspot Chi controls DNA translocation by the RecBCD helicase". Cell 114 (5): 647–654. doi:10.1016/S0092-8674(03)00681-0. PMID 13678587.
- ^ Spies, M; Amitani, I; Baskin, RJ; Kowalczykowski, Stephen C.; Kowalczykowski, SC (November 2007). "RecBCD enzyme switches lead motor subunits in response to Chi recognition". Cell 131 (4): 694–705. doi:10.1016/j.cell.2007.09.023. PMC 2151923. PMID 18022364. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2151923.
- ^ Savir Y & Tlusty T (2010). "RecA-mediated homology search as a nearly optimal signal detection system". Molecular Cell 40 (3): 388–96. doi:10.1016/j.molcel.2010.10.020. PMID 21070965. http://www.weizmann.ac.il/complex/tlusty/papers/MolCell2010.pdf.
- ^ Rambo RP, Williams GJ, Tainer JA. (2010). "Achieving fidelity in homologous recombination despite extreme complexity: informed decisions by molecular profiling". Molecular Cell 40 (3): 347–48. doi:10.1016/j.molcel.2010.10.032. PMID 21070960. http://www.weizmann.ac.il/complex/tlusty/papers/MolCell2010B.pdf.
- ^ Alberts B et al. (2008). Molecular Biology of the Cell (5th ed.). Garland Science. p. 307. ISBN 978-0-8153-4105-5.
- ^ Morimatsu, K; Kowalczykowski, SC (22 May 2003). "RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair". Molecular Cell 11 (5): 1337–1347. doi:10.1016/S1097-2765(03)00188-6. PMID 12769856.
- ^ Hiom, K (July 2009). "DNA Repair: Common Approaches to Fixing Double-Strand Breaks". Current Biology 19 (13): R523–R525. doi:10.1016/j.cub.2009.06.009. PMID 19602417.
- ^ Handa, N; Morimatsu, K; Lovett, ST; Kowalczykowski, SC (15 May 2009). "Reconstitution of initial steps of dsDNA break repair by the RecF pathway of E. coli". Genes and Development 23 (10): 1234–1245. doi:10.1101/gad.1780709. PMC 2685532. PMID 19451222. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2685532.
- ^ a b West, SC (July 2003). "Molecular views of recombination proteins and their control". Nature Reviews Molecular Cell Biology 4 (6): 435–437. doi:10.1038/nrm1127. PMID 12778123.
- ^ a b c d Watson, JD et al (2003). Molecular Biology of the Gene (5th ed.). Pearson/Benjamin Cummings. pp. 259–291. ISBN 978-0805346350.
- ^ Gumbiner-Russo, LM; Rosenberg, SM; Sandler, Steve (28 November 2007). Sandler, Steve. ed. "Physical analyses of E. coli heteroduplex recombination products in vivo: on the prevalence of 5′ and 3′ patches". PLoS One 2 (11): e1242. doi:10.1371/journal.pone.0001242. PMC 2082072. PMID 18043749. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2082072.
- ^ Thomas, CM; Nielson, KM (September 2005). "Mechanisms of, and barriers to, horizontal gene transfer between bacteria". Nature Reviews Microbiology 3 (9): 711–721. doi:10.1038/nrmicro1234. PMID 16138099. http://molecular.biosciences.wsu.edu/academics/courses/MBioS426/426-6c%20Thomas%20and%20Nielsen.pdf.
- ^ Vulic, M; Dionisio, F; Taddei, F; Radman, M (2 September 1997). "Molecular keys to speciation: DNA polymorphism and the control of genetic exchange in enterobacteria". Proceedings of the National Academy of Sciences USA 94 (18): 9763–9767. doi:10.1073/pnas.94.18.9763. PMC 23264. PMID 9275198. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=23264.
- ^ Majewski, J; Cohan, FM (January 1998). "The effect of mismatch repair and heteroduplex formation on sexual isolation in Bacillus". Genetics 48 (1): 13–18. PMC 1459767. PMID 9475717. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1459767.
- ^ Majewski, J; Zawadzki, P; Pickerill, P; Cohan, FM; Dowson, CG (February 2000). "Barriers to genetic exchange between bacterial species: Streptococcus pneumoniae transformation". Journal of Bacteriology 182 (4): 1016–1023. doi:10.1128/JB.182.4.1016-1023.2000. PMC 94378. PMID 10648528. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=94378.
- ^ Fleischmann Jr, WR (1996). "43". Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. ISBN 0-9631172-1-1. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mmed&part=A2330.
- ^ Boni, MF; de Jong, MD; van Doorn, HR; Holmes, EC; Martin, Darren P. (3 May 2010). Martin, Darren P.. ed. "Guidelines for identifying homologous recombination events in influenza a virus". PLoS One 5 (5): e10434. doi:10.1371/journal.pone.0010434. PMC 2862710. PMID 20454662. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2862710.
- ^ a b Nagy, PD; Bujarski, JJ (January 1996). "Homologous RNA recombination in brome mosaic virus: AU-rich sequences decrease the accuracy of crossovers". Journal of Virology 70 (1): 415–426. PMC 189831. PMID 8523555. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=189831.
- ^ a b Roossinck, MJ (September 1997). "Mechanisms of plant virus evolution". Annual Review of Phytopathology 35: 191–209. doi:10.1146/annurev.phyto.35.1.191. PMID 15012521.
- ^ Lamb, NE; Yu, K; Shaffer, J; Feingold, E; Sherman, SL (January 2005). "Association between maternal age and meiotic recombination for trisomy 21". The American Journal of Human Genetics 76 (1): 91–99. doi:10.1086/427266. PMC 1196437. PMID 15551222. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1196437.
- ^ Cold Spring Harbor Laboratory (2007). "Human RecQ Helicases, Homologous Recombination And Genomic Instability". ScienceDaily. http://www.sciencedaily.com/releases/2007/11/071114121320.htm. Retrieved 3 July 2010.
- ^ Modesti, M; Kanaar, R (2001). "Homologous recombination: from model organisms to human disease". Genome Biology 2 (5): REVIEWS1014. doi:10.1186/gb-2001-2-5-reviews1014. PMC 138934. PMID 11387040. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=138934.
- ^ Luo, G; Santoro, IM; McDaniel, LD; Nishijima, I; Mills, M; Youssoufian, H; Vogel, H; Schultz, RA et al. (December 2000). "Cancer predisposition caused by elevated mitotic recombination in Bloom mice". Nature Genetics 26 (4): 424–429. doi:10.1038/82548. PMID 11101838.
- ^ a b Alberts et al, B (2007). Molecular Biology of the Cell (5th ed.). Garland Science. ISBN 978-0815341109.
- ^ a b c Powell, SN; Kachnic, LA (September 2003). "Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation". Oncogene 22 (37): 5784–5791. doi:10.1038/sj.onc.1206678. PMID 12947386.
- ^ a b c d e Lin, Z; Kong, H; Nei, M; Ma, H (5 July 2006). "Origins and evolution of the recA/Rad51 gene family: evidence for ancient gene duplication and endosymbiotic gene transfer". Proceedings of the National Academy of Sciences USA 103 (27): 10328–10333. doi:10.1073/pnas.0604232103. PMC 1502457. PMID 16798872. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1502457.
- ^ Jain, SK; Cox, MM; Inman, RB (12 August 1994). "On the role of ATP hydrolysis in RecA protein-mediated DNA strand exchange. III. Unidirectional branch migration and extensive hybrid DNA formation". Journal of Biological Chemistry 269 (32): 20653–20661. PMID 8051165.
- ^ Ramesh, MA; Malik, SB; Logsdon Jr, JM (26 January 2005). "A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis". Current Biology 15 (2): 185–191. doi:10.1016/j.cub.2005.01.003. PMID 15668177.
- ^ a b Malik, SB; Ramesh, MA; Hulstrand, AM; Logsdon Jr, JM (December 2007). "Protist homologs of the meiotic Spo11 gene and topoisomerase VI reveal an evolutionary history of gene duplication and lineage-specific loss". Molecular Biology and Evolution 24 (12): 2827–2841. doi:10.1093/molbev/msm217. PMID 17921483.
- ^ Lodish H et al. (2000). "8.5:Gene Replacement and Transgenic Animals: DNA Is Transferred into Eukaryotic Cells in Various Ways". Molecular Cell Biology (4th ed.). W. H. Freeman and Company. ISBN 0-7167-3136-3.
- ^ "The Nobel Prize in Physiology or Medicine 2007". The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/2007/index.html. Retrieved December 15, 2008.
- ^ Drummond, DA; Silberg, JJ; Meyer, MM; Wilke, CO; Arnold, FH (12 April 2005). "On the conservative nature of intragenic recombination". Proceedings of the National Academy of Sciences USA 102 (15): 5380–5385. doi:10.1073/pnas.0500729102. PMC 556249. PMID 15809422. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=556249.
- ^ Bloom, JD; Silberg, JJ; Wilke, CO; Drummond, DA; Adami, C; Arnold, FH (15 January 2005). "Thermodynamic prediction of protein neutrality". Proceedings of the National Academy of Sciences USA 102 (3): 606–611. doi:10.1073/pnas.0406744102. PMC 545518. PMID 15644440. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=545518.
- ^ a b Carbone, MN; Arnold, FH (August 2007). "Engineering by homologous recombination: exploring sequence and function within a conserved fold". Current Opinion in Structural Biology 17 (4): 454–459. doi:10.1016/j.sbi.2007.08.005. PMID 17884462.
- ^ Otey, CR; Landwehr, M; Endelman, JB; Hiraga, K; Bloom, JD; Arnold, FH (May 2006). "Structure-guided recombination creates an artificial family of cytochromes P450". PLoS Biology 4 (5): e112. doi:10.1371/journal.pbio.0040112. PMC 1431580. PMID 16594730. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1431580.
- ^ Socolich, M; Lockless, SW; Russ, WP; Lee, H; Gardner, KH; Ranganathan, R (22 September 2005). "Evolutionary information for specifying a protein fold". Nature 437 (7058): 512–518. doi:10.1038/nature03991. PMID 16177782.
- ^ Thulasiram, HV; Erickson, HK; Poulter, CD (6 April 2007). "Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis". Science 316 (5821): 73–76. doi:10.1126/science.1137786. PMID 17412950.
- ^ Landwehr, M; Carbone, M; Otey, CR; Li, Y; Arnold, FH (March 2007). "Diversification of catalytic function in a synthetic family of chimeric cytochrome P450s". Chemistry and Biology 14 (3): 269–278. doi:10.1016/j.chembiol.2007.01.009. PMC 1991292. PMID 17379142. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1991292.
- ^ a b c d Iglehart, JD; Silver, DP (9 July 2009). "Synthetic lethality — a new direction in cancer-drug development". New England Journal of Medicine 361 (2): 189–191. doi:10.1056/NEJMe0903044. PMID 19553640.
- ^ Fong, FC; Boss, DS; Yap, TA; Tutt, A; Wu, P; Mergui-Roelvink, M; Mortimer, P; Swaisland, H et al. (9 July 2009). "Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers". New England Journal of Medicine 361 (2): 123–134. doi:10.1056/NEJMoa0900212. PMID 19553641.
- ^ Edwards, SL; Brough, R; Lord, CJ; Natrajan, R; Vatcheva, R; Levine, DA; Boyd, J; Reis-Filho, JS et al. (28 February 2008). "Resistance to therapy caused by intragenic deletion in BRCA2". Nature 451 (7182): 1111–1115. doi:10.1038/nature06548. PMID 18264088.
External links
- Animations – homologous recombination: Animations showing several models of homologous recombination
- Homologous recombination: Tempy & Trun: Animation of the bacterial RecBCD pathway of homologous recombination
Genetics: homologous recombination / mobile genetic elements Primarily prokaryotic Occurs in eukaryotes Categories:
Wikimedia Foundation. 2010.