- Sodium channel
-
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's plasma membrane.[1][2] They are classified according to the trigger that opens the channel for such ions, i.e. either a voltage-change (voltage-gated sodium channels) or binding of a substance (a ligand) to the channel (ligand-gated sodium channels).
In excitable cells such as neurons, myocytes, and certain types of glia, sodium channels are responsible for the rising phase of action potentials.
Contents
Voltage-gated
Structure
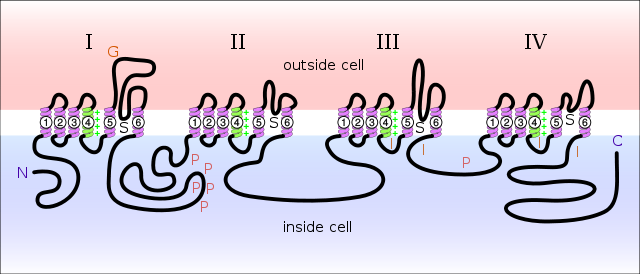 Diagram of a voltage-sensitive sodium channel α-subunit. G – glycosylation, P – phosphorylation, S – ion selectivity, I – inactivation, positive (+) charges in S4 are important for transmembrane voltage sensing.[3]
Diagram of a voltage-sensitive sodium channel α-subunit. G – glycosylation, P – phosphorylation, S – ion selectivity, I – inactivation, positive (+) charges in S4 are important for transmembrane voltage sensing.[3]
Sodium channels consist of a large α subunit that associates with other proteins, such as β subunits. An α subunit forms the core of the channel and is functional on its own. When the α subunit protein is expressed by a cell, it is able to form channels that conduct Na+ in a voltage-gated way, even if β subunits or other known modulating proteins are not expressed. When accessory proteins assemble with α subunits, the resulting complex can display altered voltage dependence and cellular localization.
The α-subunit has four repeat domains, labeled I through IV, each containing six membrane-spanning regions, labeled S1 through S6. The highly conserved S4 region acts as the channel's voltage sensor. The voltage sensitivity of this channel is due to positive amino acids located at every third position. When stimulated by a change in transmembrane voltage, this region moves toward the extracellular side of the cell membrane, allowing the channel to become permeable to ions. The ions are conducted through a pore, which can be broken into two regions. The more external (i.e., more extracellular) portion of the pore is formed by the "P-loops" (the region between S5 and S6) of the four domains. This region is the most narrow part of the pore and is responsible for its ion selectivity. The inner portion (i.e., more cytoplasmic) of the pore is formed by the combined S5 and S6 regions of the four domains. The region linking domains III and IV is also important for channel function. This region plugs the channel after prolonged activation, inactivating it.
Gating
Voltage-gated sodium channels have three types of states: deactivated (closed), activated (open), and inactivated (closed). Channels in the deactivated state are thought to be blocked on their intracellular side by an "activation gate", which is removed in response to stimulation that opens the channel. The ability to inactivate is thought to be due to a tethered plug (formed by domains III and IV of the alpha subunit), called an inactivation gate, that blocks the inside of the channel shortly after it has been activated. During an action potential the channel remains inactivated for a few milliseconds after depolarization. The inactivation is removed when the membrane potential of the cell repolarizes following the falling phase of the action potential. This allows the channels to be activated again during the next action potential. Genetic diseases that alter sodium channel inactivation can cause muscle stiffness or epileptic seizures because of the introduction of a so-called window current, during which sodium channels are tonically active, causing muscle and/or nerve cells to become over-excited.
The temporal behaviour of sodium channels can be modeled by a Markovian scheme or by the Hodgkin-Huxley-type formalism. In the former scheme, each channel occupies a distinct state with differential equations describing transitions between states; in the latter, the channels are treated as a population that are affected by three independent gating variables. Each of these variables can attain a value between 1 (fully permeant to ions) and 0 (fully non-permeant), the product of these variables yielding the percentage of conducting channels. The Hodgkin-Huxley model can be shown to be equivalent to a Markovian model.
Impermeability to other ions
The pore of sodium channels contains a selectivity filter made of negatively charged amino acid residues, which attract the positive Na+ ion and keep out negatively charged ions such as chloride. The cations flow into a more constricted part of the pore that is 0.3 by 0.5 nm wide, which is just large enough to allow a single Na+ ion with a water molecule associated to pass through. The larger K+ ion cannot fit through this area. Differently sized ions also cannot interact as well with the negatively charged glutamic acid residues that line the pore.
Diversity
Voltage-gated sodium channels normally consist of an alpha subunit that forms the ion conduction pore and one to two beta subunits that have several functions including modulation of channel gating.[4] Expression of the alpha subunit alone is sufficient to produce a functional channel.
Alpha subunits
The family of sodium channels has nine known members, with amino acid identity >50% in the transmembrane and extracellular loop regions. A standardized nomenclature for sodium channels is currently used and is maintained by the IUPHAR.[5][6]
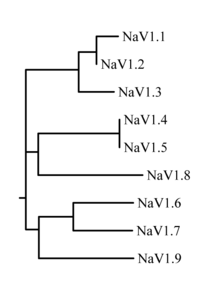
The proteins of these channels are named Nav1.1 through Nav1.9. The gene names are referred to as SCN1A through SCN11A (the SCN6/7A gene is part of the Nax sub-family and has uncertain function). The likely evolutionary relationship between these channels, based on the similarity of their amino acid sequences, is shown in figure 1. The individual sodium channels are distinguished not only by differences in their sequence but also by their kinetics and expression profiles. Some of this data is summarized in table 1, below.
Table 1. Nomenclature and some functions of voltage-gated sodium channel alpha subunits Protein name Gene Expression profile Associated human channelopathies Nav1.1 SCN1A Central neurons, [peripheral neurons] and cardiac myocytes febrile epilepsy, GEFS+, Dravet syndrome (also known as severe myclonic epilepsy of infancy or SMEI), borderline SMEI (SMEB), West syndrome (also known as infantile spasms), Doose syndrome (also known as myoclonic astatic epilepsy), intractable childhood epilepsy with generalized tonic-clonic seizures (ICEGTC), Panayiotopoulos syndrome, familial hemiplegic migraine (FHM), familial autism, Rasmussens's encephalitis and Lennox-Gastaut syndrome[7] Nav1.2 SCN2A Central neurons, peripheral neurons inherited febrile seizures and epilepsy Nav1.3 SCN3A Central neurons, peripheral neurons and cardiac myocytes none known Nav1.4 SCN4A Skeletal muscle hyperkalemic periodic paralysis, paramyotonia congenita, and potassium-aggravated myotonia Nav1.5 SCN5A Cardiac myocytes, uninnervated skeletal muscle, central neurons Long QT syndrome, Brugada syndrome, and idiopathic ventricular fibrillation Nav1.6 SCN8A Central neurons, dorsal root ganglia, peripheral neurons, heart, glia cells none known Nav1.7 SCN9A Dorsal root ganglia, sympathetic neurons, Schwann cells, and neuroendocrine cells erythromelalgia, PEPD and channelopathy-associated insensitivity to pain Nav1.8 SCN10A Dorsal root ganglia none known Nav1.9 SCN11A Dorsal root ganglia none known Nax SCN7A heart, uterus, skeletal muscle, astrocytes, dorsal root ganglion cells none known Beta subunits
Sodium channel beta subunits are type 1 transmembrane glycoproteins with an extracellular N-terminus and a cytoplasmic C-terminus. As a member of the Ig superfamily, beta subunits contain a prototypic V-set Ig loop in their extracellular domain. Interestingly, beta subunits share no homology with their counterparts of calcium and potassium channels.[8] Instead, they are homologous to neural cell adhesion molecules (CAMs) and the large family of L1 CAMs. There are four distinct betas named in order of discovery: SCN1B, SCN2B, SCN3B, SCN4B (table 2). Beta 1 and beta 3 interact with the alpha subunit non-covalently while beta 2 and beta 4 associate with alpha via disulfide bond.[9]
Role of beta subunits as cell adhesion molecules
In addition to regulating channel gating, sodium channel beta subunits also modulate channel expression and form links to the intracelluar cytoskeleton via ankyrin and spectrin.[4][10][11] Voltage-gated sodium channels also assemble with a variety of other proteins, such as FHF proteins (Fibroblast growth factor Homologous Factor), calmodulin, cytoskeleton or regulatory kinases,[12][13][14][15][16] which form a complex with sodium channels, influencing its expression and/or function. Several beta subunits interact with one or more extracellular matrix (ECM) molecules. Contactin, also known as F3 or F11, associates with beta 1 as shown via co-immunoprecipitation.[17] Fibronectin-like (FN-like) repeats of Tenascin-C and Tenascin-R bind with beta 2 in contrast to the Epidermal growth factor-like (EGF-like) repeats that repel beta2.[18] A disintegrin and metalloproteinase (ADAM) 10 sheds beta 2's ectodomain possibly inducing neurite outgrowth.[19] Beta 3 and beta 1 bind to neurofascin at Nodes of Ranvier in developing neurons.[20]
Table 2. Nomenclature and some functions of voltage-gated sodium channel beta subunits Protein name Gene link Assembles with Expression profile Associated human channelopathies Navβ1 SCN1B Nav1.1 to Nav1.7 Central Neurons, Peripheral Neurons, skeletal muscle, heart, glia epilepsy (GEFS+) Navβ2 SCN2B Nav1.1, Nav1.2, Nav1.5 to Nav1.7 Central Neurons, peripheral neurons, heart, glia none known Navβ3 SCN3B Nav1.1 to Nav1.3, Nav1.5 central neurons, adrenal gland, kidney, peripheral neurons none known Navβ4 SCN4B Nav1.1, Nav1.2, Nav1.5 heart, skeletal muscle, central and peripheral neurons none known Ligand-gated
Ligand-gated sodium channels are activated by binding of a ligand instead of a change in membrane potential.
They are found e.g. in the neuromuscular junction as nicotinic receptors, where the ligands are acetylcholine molecules. Most channels of this type are permeable to potassium to some degree as well as to sodium.
Role in action potential
- See main article: Action potential
Voltage-gated sodium channels play an important role in action potentials. If enough channels open when there is a change in the cell's membrane potential, a small but significant number of Na+ ions will move into the cell down their electrochemical gradient, further depolarizing the cell. Thus, the more Na+ channels localized in a region of a cell's membrane, the faster the action potential will propagate, and the more excitable that area of the cell will be. This is an example of a positive feedback loop. The ability of these channels to assume a closed-inactivated state causes the refractory period and is critical for the propagation of action potentials down an axon.
Na+ channels both open and close more quickly than K+ channels, producing an influx of positive charge (Na+) toward the beginning of the action potential and an efflux (K+) toward the end.
Ligand-gated sodium channels, on the other hand, create the change in the membrane potential in the first place, in response to the binding of a ligand to it.
Pharmacologic modulation
Blockers
See Sodium channel blockers
Activators
The following naturally produced substances persistently activate (open) sodium channels:
- Alkaloid based toxins
Gating modifiers
The following toxins modify the gating of sodium channels:
See also
- Resting ion channels
- Epithelial sodium channel
References
- ^ Jessell TM, Kandel ER, Schwartz JH (2000). Principles of Neural Science (4th ed.). New York: McGraw-Hill. pp. 154–69. ISBN 0-8385-7701-6.
- ^ Bertil Hillel (2001). Ion Channels of Excitable Membranes (3rd ed.). Sunderland, Mass: Sinauer. pp. 73–7. ISBN 0-87893-321-2.
- ^ Yu FH, Catterall WA (2003). "Overview of the voltage-gated sodium channel family". Genome Biol 4 (3): 207. doi:10.1186/gb-2003-4-3-207. PMC 153452. PMID 12620097. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=153452.
- ^ a b Isom LL (2001). "Sodium channel beta subunits: anything but auxiliary". Neuroscientist 7 (1): 42–54. doi:10.1177/107385840100700108. PMID 11486343.
- ^ IUPHAR – International Union of Basic and Clinical Pharmacology
- ^ Catterall WA, Goldin AL, Waxman SG (2005). "International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels". Pharmacol Rev 57 (4): 397–409. doi:10.1124/pr.57.4.4. PMID 16382098.
- ^ Lossin C. "SCN1A infobase". http://www.scn1a.info/. Retrieved 2009-10-30. "compilation of genetic variations in the SCN1A gene that alter the expression or function of Nav1.1"
- ^ Catterall WA (April 2000). "From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels". Neuron 26 (1): 13–25. doi:10.1016/S0896-6273(00)81133-2. PMID 10798388.
- ^ Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA (May 1992). "Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel". Science 256 (5058): 839–42. doi:10.1126/science.1375395. PMID 1375395.
- ^ Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL (April 2000). "Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact". J. Biol. Chem. 275 (15): 11383–8. doi:10.1074/jbc.275.15.11383. PMID 10753953.
- ^ Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL (July 2002). "Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin". J. Biol. Chem. 277 (29): 26681–8. doi:10.1074/jbc.M202354200. PMID 11997395.
- ^ Cantrell AR, Catterall WA (June 2001). "Neuromodulation of Na+ channels: an unexpected form of cellular plasticity". Nat. Rev. Neurosci. 2 (6): 397–407. doi:10.1038/35077553. PMID 11389473.
- ^ Isom LL (February 2001). "Sodium channel beta subunits: anything but auxiliary". Neuroscientist 7 (1): 42–54. doi:10.1177/107385840100700108. PMID 11486343.
- ^ Shah BS, Rush AM, Liu S, Tyrrell L, Black JA, Dib-Hajj SD, Waxman SG (August 2004). "Contactin associates with sodium channel Nav1.3 in native tissues and increases channel density at the cell surface". J. Neurosci. 24 (33): 7387–99. doi:10.1523/JNEUROSCI.0322-04.2004. PMID 15317864.
- ^ Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD (July 2004). "Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of Ranvier of dorsal root axons". J. Neurosci. 24 (30): 6765–75. doi:10.1523/JNEUROSCI.1628-04.2004. PMID 15282281.
- ^ Rush AM, Wittmack EK, Tyrrell L, Black JA, Dib-Hajj SD, Waxman SG (May 2006). "Differential modulation of sodium channel Na(v)1.6 by two members of the fibroblast growth factor homologous factor 2 subfamily". Eur. J. Neurosci. 23 (10): 2551–62. doi:10.1111/j.1460-9568.2006.04789.x. PMID 16817858.
- ^ Kazarinova-Noyes, K., et al., Contactin associates with Na+ channels and increases their functional expression. J Neurosci, 2001. 21(19): p. 7517-25
- ^ Srinivasan J, Schachner M, Catterall WA (December 1998). "Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R". Proc. Natl. Acad. Sci. U.S.A. 95 (26): 15753–7. doi:10.1073/pnas.95.26.15753. PMC 28116. PMID 9861042. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=28116.
- ^ Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM (June 2005). "Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration". J. Biol. Chem. 280 (24): 23251–61. doi:10.1074/jbc.M412938200. PMID 15833746.
- ^ Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA (July 2001). "Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain". J. Cell Biol. 154 (2): 427–34. doi:10.1083/jcb.200102086. PMC 2150779. PMID 11470829. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2150779.
- ^ Grolleau F, Stankiewicz M, Birinyi-Strachan L, Wang XH, Nicholson GM, Pelhate M, Lapied B (2001). "Electrophysiological analysis of the neurotoxic action of a funnel-web spider toxin, delta-atracotoxin-HV1a, on insect voltage-gated Na+ channels". J. Exp. Biol. 204 (Pt 4): 711–21. PMID 11171353.
- ^ Possani, L.D.; Becerrill, B.; Delepierre, M.; Tytgat Hammock, J. (1999). "Scorpion toxins specific for Na+-channels". European Journal of Biochemistry 264 (2): 287–300. doi:10.1046/j.1432-1327.1999.00625.x. PMID 10491073.
External links
- MeSH Sodium+Channels
- "Voltage-Gated Sodium Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. http://www.iuphar-db.org/IC/FamilyMenuForward?familyId=10.
Ca2+: Calcium channel Ligand-gatedNa+: Sodium channel Constitutively activeProton gatedK+: Potassium channel Kvα1-6 (1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8) · (2.1, 2.2) · (3.1, 3.2, 3.3, 3.4) · (4.1, 4.2, 4.3) · (5.1) · (6.1, 6.2, 6.3, 6.4)
Kvα7-12 (7.1, 7.2, 7.3, 7.4, 7.5) · (8.1, 8.2) · (9.1, 9.2, 9.3) · (10.1, 10.2) · (11.1/hERG, 11.2, 11.3) · (12.1, 12.2, 12.3)
Kvβ (1, 2, 3) · KCNIP (1, 2, 3, 4) · minK/ISK · minK/ISK-like · MiRP (1, 2, 3) · Shaker geneOther Cl-: Chloride channelHVCN1Generalsee also disorders
B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othrCategories:- Ion channels
- Electrophysiology
- Sodium
- Integral membrane proteins
Wikimedia Foundation. 2010.