- Leptin
-
Leptin 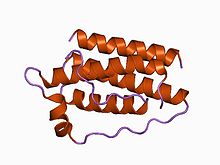
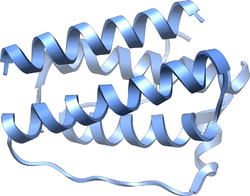
Structure of the obese protein leptin-E100.[1] Identifiers Symbol Leptin Pfam PF02024 Pfam clan CL0053 InterPro IPR000065 SCOP 1ax8 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Leptin (Greek leptos meaning thin) is a 16 kDa protein hormone that plays a key role in regulating energy intake and energy expenditure, including appetite and metabolism. It is one of the most important adipose derived hormones.[2] The Ob(Lep) gene (Ob for obese, Lep for leptin) is located on chromosome 7 in humans.[3]
Contents
Discovery
The effects of leptin were observed by studying mutant obese mice that arose at random within a mouse colony at the Jackson Laboratory in 1950.[4] These mice were massively obese and excessively voracious. Ultimately, several strains of laboratory mice have been found to be homozygous for single-gene mutations that cause them to become grossly obese, and they fall into two classes: "ob/ob", those having mutations in the gene for the protein hormone leptin, and "db/db", those having mutations in the gene that encodes the receptor for leptin. When ob/ob mice are treated with injections of leptin, they lose their excess fat and return to normal body weight.
Leptin itself was discovered in 1994 by Jeffrey M. Friedman and colleagues at the Rockefeller University through the study of such mice.[5]
Biosynthesis
Human leptin is a protein of 167 amino acids. It is manufactured primarily in the adipocytes of white adipose tissue, and the level of circulating leptin is directly proportional to the total amount of fat in the body.
In addition to white adipose tissue—the major source of leptin—it can also be produced by brown adipose tissue, placenta (syncytiotrophoblasts), ovaries, skeletal muscle, stomach (lower part of fundic glands), mammary epithelial cells, bone marrow, pituitary and liver.[6]
Leptin has also been discovered to be synthesised from gastric chief cells and P/D1 cells in the stomach.[7]
Function
Leptin acts on receptors in the hypothalamus of the brain where it inhibits appetite by (1) counteracting the effects of neuropeptide Y (a potent feeding stimulant secreted by cells in the gut and in the hypothalamus); (2) counteracting the effects of anandamide (another potent feeding stimulant that binds to the same receptors as THC), and (3) promoting the synthesis of α-MSH, an appetite suppressant. This inhibition is long-term, in contrast to the rapid inhibition of eating by cholecystokinin (CCK) and the slower suppression of hunger between meals mediated by PYY3-36. The absence of leptin (or its receptor) leads to uncontrolled food intake and resulting obesity. Several studies have shown that fasting or following a very-low-calorie diet (VLCD) lowers leptin levels.[8] It might be that, in the short-term, leptin is an indicator of energy balance. This system is more sensitive to starvation than to overfeeding; leptin levels change more when food intake decreases than when it increases.[9] It might be that the dynamics of leptin due to an acute change in energy balance are related to appetite and eventually to food intake. Although this is a new hypothesis, there are already some data that support it.[10][11]
There is some controversy regarding the regulation of leptin by melatonin during the night. One research group suggested that increased levels of melatonin caused a downregulation of leptin.[12] However, in 2004, Brazilian researchers found that melatonin increases leptin levels in the presence of insulin, therefore causing a decrease in appetite during sleeping.[13]
Mice with type 1 diabetes treated with leptin alone or in conjunction with insulin did better (blood sugar did not fluctuate as much; cholesterol levels decreased; mice formed less body fat) than mice with type 1 diabetes treated with insulin alone, raising the prospect of a new treatment for diabetes.[14]
Adiposity signal
To date, only leptin and insulin are known to act as an adiposity signal. In general,
- Leptin circulates at levels proportional to body fat.
- It enters the central nervous system (CNS) in proportion to its plasma concentration.
- Its receptors are found in brain neurons involved in regulating energy intake and expenditure.
- It controls food intake and energy expenditure by acting on receptors in the mediobasal hypothalamus[15]
Interaction with amylin
Co-administration of two neurohormones known to have a role in body weight control, amylin (produced by beta cells in the pancreas) and leptin (produced by fat cells), results in sustained, fat-specific weight loss in a leptin-resistant animal model of obesity.[16]
Satiety
Leptin binds to neuropeptide Y (NPY) neurons in the arcuate nucleus, in such a way that decreases the activity of these neurons. Leptin signals to the brain that the body has had enough to eat, producing a feeling of satiety. A very small group of humans possess homozygous mutations for the leptin gene that leads to a constant desire for food, resulting in severe obesity. This condition can be treated somewhat successfully by the administration of recombinant human leptin.[17] However, extensive clinical trials using recombinant human leptin as a therapeutic agent for treating obesity in humans have been inconclusive because only the most obese subjects who were given the highest doses of exogenous leptin produced statistically significant weight loss. It was concluded that large and frequent doses are needed to provide only modest benefit because of leptin’s low circulating half-life, low potency, and poor solubility. Furthermore, these injections caused some participants to drop out of the study due to inflammatory responses of the skin at the injection site. Some of these problems can be alleviated by a form of leptin called Fc-leptin, which takes the Fc fragment from the immunoglobulin gamma chain as the N-terminal fusion partner and follows it with leptin. This Fc-leptin fusion has been experimentally proven to be highly soluble, more biologically potent, and contain a much longer serum half-life. As a result, this Fc-leptin was successfully shown to treat obesity in both leptin-deficient and normal mice, although studies have not been undertaken on human subjects. This makes Fc-leptin a potential treatment for obesity in humans after more extensive testing.[18][19][20] Circulating leptin levels give the brain input regarding energy storage so it can regulate appetite and metabolism. Leptin works by inhibiting the activity of neurons that contain neuropeptide Y (NPY) and agouti-related peptide (AgRP), and by increasing the activity of neurons expressing α-melanocyte-stimulating hormone (α-MSH). The NPY neurons are a key element in the regulation of appetite; small doses of NPY injected into the brains of experimental animals stimulates feeding, while selective destruction of the NPY neurons in mice causes them to become anorexic. On the converse, α-MSH is an important mediator of satiety, and differences in the gene for the receptor at which α-MSH acts in the brain are linked to obesity in humans.
Circulatory system
The role of leptin/leptin receptors in modulation of T cell activity in immune system was shown in experimentation with mice. It modulates the immune response to atherosclerosis, which is a predisposing factor in patients with obesity.[21]
Leptin promotes angiogenesis by increasing vascular endothelial growth factor (VEGF) levels.
In some epidemiological studies, hyperleptinemia is considered as a risk factor. However, recently a handful of animal experiments demonstrated that systemic hyperleptinemia produced by infusion or adenoviral gene transfer decreases blood pressure in rats.[22][23]
Lung surfactant activity
In fetal lung, leptin is induced in the alveolar interstitial fibroblasts ("lipofibroblasts") by the action of PTHrP secreted by formative alveolar epithelium (endoderm) under moderate stretch. The leptin from the mesenchyme, in turn, acts back on the epithelium at the leptin receptor carried in the alveolar type II pneumocytes and induces surfactant expression, which is one of the main functions of these type II pneumocytes.[24]
Reproduction
In mice, leptin is also required for male and female fertility. Leptin has a lesser effect in humans. In mammals such as humans, ovulatory cycles in females are linked to energy balance (positive or negative depending on whether a female is losing or gaining weight) and energy flux (how much energy is consumed and expended) much more than energy status (fat levels). When energy balance is highly negative (meaning that a woman is starving) or energy flux is very high (meaning that a woman is exercising at extreme levels, but still consuming enough calories), the ovarian cycle stops and females stop menstruating. Only if a female has an extremely low body fat percentage does energy status affect menstruation. Some studies have indicated that leptin levels outside an ideal range can have a negative effect on egg quality and outcome during IVF.[25]
The body's fat cells, under normal conditions, are responsible for the constant production and release of leptin. This can also be produced by the placenta.[26] Leptin levels rise during pregnancy and fall after parturition (childbirth). Leptin is also expressed in fetal membranes and the uterine tissue. Uterine contractions are inhibited by leptin.[27]
There is also evidence that leptin plays a role in hyperemesis gravidarum (severe morning sickness of pregnancy),[28] in polycystic ovary syndrome[29] and a 2007 research suggests that hypothalamic leptin is implicated in bone growth.[30]
Effects on bone
The fact that leptin, a hormone released from fat tissue, can regulate bone mass first came to prominence in 2000.[31] It is now well established that leptin can affect bone metabolism via direct signalling from the brain and that although leptin acts to reduce cancellous bone, it conversely increases cortical bone. A number of theories have been put forward concerning the cortical-cancellous dichotomy including a recent theory suggesting that increased leptin during obesity may represent a mechanism for enlarging bone size and thus bone resistance to cope with increased body weight.[32]
Bone metabolism is under direct control of the brain and thus nerve fibres are present in bone tissue.[33] A number of brain signalling molecules (neuropeptides and neurotransmitters) have been found in bone including adrenaline, noradrenaline, serotonin, calcitonin gene-related peptide, vasoactive intestinal peptide and neuropeptide Y.[33][34] This evidence supports a direct signalling system between the brain and bone with accumulating evidence suggesting that these molecules are directly involved in the regulation of bone metabolism. Leptin, once released from fat tissue, can cross the blood-brain barrier and bind to its receptors in the brain where it acts through the sympathetic nervous system to regulate bone metabolism.[35] It is also possible that, in addition to its effects through the brain, leptin may act directly on cells in the bone to regulate bone metabolism. In reality, leptin probably signals to bone on multiple levels, with local and systemic checks and balances impacting the final outcome. As a result, the clinical utility of leptin for treatment of bone diseases remains open but ongoing research may yet provide much needed therapies for stimulating bone formation.
Clinical significance
Leptin has traditionally been regarded as a link between fat mass, food intake, and energy expenditure. This link originally arose from animal research findings, but its application to describing human systems has since been challenged.[36] In humans, there are many instances where leptin dissociates from the strict role of communicating nutritional status between body and brain and no longer correlates with body fat levels:
- Leptin levels decrease after short-term fasting (24–72 hours), even when changes in fat mass are not observed.[37]
- In the obese patients with obstructive sleep apnea (OSA), Leptin is increased, but decreases after administration of a CPAP.[38][39] In non-obese individuals, however, restful sleep (i.e., 8–12 hours of unbroken sleep) can increase leptin within normal ranges.
- Serum levels of Leptin are reduced by sleep deprivation.[40][41]
- Increased by perceived emotional stress.[42]
- Decreased by testosterone and increased by estrogen.[43]
- Chronically affected by exercise training; it decreases leptin levels.[44]
Inflammatory marker
Factors that acutely affect leptin levels are also factors that influence other markers of inflammation, e.g., testosterone, sleep, emotional stress, caloric restriction, and body fat levels. While it is well-established that leptin is involved in the regulation of the inflammatory response,[45][46][47] it has been further theorized that leptin's role as an inflammatory marker is to respond specifically to adipose-derived inflammatory cytokines.
In terms of both structure and function, leptin resembles IL-6 and is a member of the cytokine superfamily.[1][46][48] Circulating leptin seems to effect the HPA axis, suggesting a role for leptin in stress response.[49] Elevated leptin concentrations are associated with elevated white blood cell counts in both men and women.[50]
Similar to what is observed in chronic inflammation, chronically-elevated leptin levels are associated with obesity, overeating, and inflammation-related diseases including hypertension, metabolic syndrome, and cardiovascular disease. However, while leptin is associated with body fat mass, the size of individual fat cells, and the act of overeating, it is interesting that it is not affected by exercise (for comparison, IL-6 is released in response to muscular contractions). Thus, it is speculated that leptin responds specifically to adipose-derived inflammation.[51] Leptin is a pro-angiogenic, pro-inflammatory and mitogenic factor, the actions of which are reinforced through crosstalk with IL-1 family cytokines in cancer. [52]
Taken as such, increases in leptin levels (in response to caloric intake) function as an acute pro-inflammatory response mechanism to prevent excessive cellular stress induced by overeating. When high caloric intake overtaxes fat cells' ability to grow larger or increase in number in step with caloric intake, the ensuing stress response leads to inflammation at the cellular level and ectopic fat storage, i.e., the unhealthy storage of body fat within internal organs, arteries, and/or muscle. The insulin increase in response to the caloric load provokes a dose-dependent rise in leptin, an effect potentiated by high cortisol levels.[53] (This insulin-leptin relationship is notably similar to insulin's effect on the increase of IL-6 gene expression and secretion from preadipocytes in a time- and dose-dependent manner.)[54] Furthermore, plasma leptin concentrations have been observed to gradually increase when acipimox is administered to prevent lipolysis, concurrent hypocaloric dieting and weight loss notwithstanding.[55] Such findings appear to demonstrate that high caloric loads in excess of fat cells' storage rate capacities lead to stress responses that induce an increase in leptin, which then operates as an adipose-derived inflammation stopgap signaling for the cessation of food intake so as to prevent adipose-derived inflammation from reaching elevated levels. This response may then protect against the harmful process of ectopic fat storage, which perhaps explains the connection between chronically-elevated leptin levels and ectopic fat storage in obese individuals.
Obesity and leptin resistance
Although leptin is a circulating signal that reduces appetite, obese individuals generally exhibit an unusually high circulating concentration of leptin.[56] These people are said to be resistant to the effects of leptin, in much the same way that people with type 2 diabetes are resistant to the effects of insulin. The high sustained concentrations of leptin from the enlarged adipose stores result in leptin desensitization. The pathway of leptin control in obese people might be flawed at some point so the body does not adequately receive the satiety feeling subsequent to eating.
Some researchers attempted to explain the failure of leptin to prevent obesity in modern humans as a metabolic disorder, possibly caused by a specific nutrient or a combination of nutrients that were not present or were not common in the prehistoric diet. Some proposed "villain" nutrients include lectins[57] and fructose.[58]
A signal-to-noise ratio theory has been proposed to explain the phenomenon of leptin resistance.[36] In healthy individuals, baseline leptin levels are between 1-5 ng/dl in men and 7-13 ng/dl in women.[36] A large intake of calories triggers a leptin response that reduces hunger, thereby preventing an overload of the inflammatory response induced by caloric intake. It has been theorized that, in obese individuals, the leptin response to caloric intake is blunted due to chronic, low-grade hyperleptinemia depressing the signal-to-noise ratio such that acute leptin responses have less of a physiological effect on the body.
Although leptin resistance is sometimes described as a metabolic disorder that contributes to obesity, similar to the way insulin resistance is sometimes described as a metabolic disorder that has the potential to progress into the type 2 diabetes, it is not certain that it is true in most cases. The mere fact that leptin resistance is extremely common in obese individuals suggests that it may simply be an adaptation to excess body weight. It has been suggested that the major physiological role of leptin is not as a “satiety signal” to prevent obesity in times of energy excess, but as a “starvation signal” to maintain adequate fat stores for survival during times of energy deficit,[59][60] and that leptin resistance in overweight individuals is the standard feature of mammalian physiology, which possibly confers a survival advantage.[61]
A different form of leptin resistance (in combination with insulin resistance and weight gain) easily arises in laboratory animals (such as rats), as soon as they are given unlimited (ad libitum) access to palatable, energy-dense foods,[62] and it is reversed when these animals are put back on low energy-density chow.[63] That, too, may have an evolutionary advantage: "the ability to efficiently store energy during periods of sporadic feast represented a survival advantage in ancestral societies subjected to periods of starvation." [64] The combination of two mechanisms (one, which temporarily suspends leptin action when presented with excess of high-quality food, and the other, which blunts the processes that could drive the body weight back to "normal"), could explain the current obesity epidemic without invoking any metabolic disorders or "villain" nutrients.
Interactions with fructose
A study published suggests that the consumption of high amounts of fructose causes leptin resistance and elevated triglycerides in rats. The rats consuming the high-fructose diet subsequently ate more and gained more weight than controls when fed a high-fat, high-calorie diet.[65][66][67] These studies however did not control against other monosaccharides or polysaccharides, therefore leptin resistance may be a result of a diet that contains high saccharide indexes (soda, candy, and other easily sugar-liberated foods).
Mechanism of action
Leptin interacts with six types of receptors (Ob-Ra–Ob-Rf, or LepRa-LepRf) that in turn are encoded by a single gene, LEPR.[68] Ob-Rb is the only receptor isoform that can signal intracellularly via the Jak-Stat and MAPK signal transduction pathways,[69] and is present in hypothalamic nuclei.[70]
It is unknown as to whether leptin can cross the blood-brain barrier to access receptor neurons, because the blood-brain barrier is attenuated in the area of the median eminence, close to where the NPY neurons of the arcuate nucleus are. It is generally thought that leptin might enter the brain at the choroid plexus, where there is intense expression of a form of leptin receptor molecule that could act as a transport mechanism.
Once leptin has bound to the Ob-Rb receptor, it activates the stat3, which is phosphorylated and travels to the nucleus to, it is presumed, effect changes in gene expression. One of the main effects on gene expression is the down-regulation of the expression of endocannabinoids, responsible for increasing appetite[citation needed]. There are other intracellular pathways activated by leptin, but less is known about how they function in this system. In response to leptin, receptor neurons have been shown to remodel themselves, changing the number and types of synapses that fire onto them.
There is some recognition that leptin action is more decentralized than previously assumed. In addition to its endocrine action at a distance (from adipose tissue to brain), leptin also acts as a paracrine mediator.[6]
Metreleptin
An analog of human leptin, metreleptin is under investigation for the treatment of diabetes and/or hypertriglyceridemia, in patients with rare forms of lipodystrophy, syndromes characterized by abnormalities in adipose tissue distribution, and severe metabolic abnormalities. Amylin Pharmaceuticals, the drug's developer, has received orphan drug designation for metreleptin from the U.S. Food and Drug Administration (FDA) for this indication, and plans to complete submission of metreleptin to the FDA by the end of 2011. In a three-year-long study of metreleptin in patients with lipodystrophy organized by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH), metreleptin treatment was associated with a significant decrease in blood glucose (A1c decrease from 9.4% at baseline to 7.0% at study end) and triglyceride concentration (from 500 mg/dL at baseline to 200 mg/dL at study end).[71]
Metreleptin is also under clinical investigation in combination with pramlintide, an analog of the hormone amylin, for the treatment of obesity. Co-administration of amylin (produced by beta cells in the pancreas) and leptin (produced by fat cells), results in sustained, fat-specific weight loss in a leptin-resistant animal model of obesity.[16]
The Juvenile Diabetes Research Foundation (JDRF) has also partnered with Amylin Pharmaceuticals and researchers at the The University of Texas (UT) Southwestern Medical Center to study whether metreleptin can be used to improve the treatment of type 1 diabetes.[72]
See also
- Teleost leptins
References
- ^ a b Zhang F, Basinski MB, Beals JM et al. (May 1997). "Crystal structure of the obese protein leptin-E100". Nature 387 (6629): 206–9. doi:10.1038/387206a0. PMID 9144295.
- ^ Brennan AM, Mantzoros CS (June 2006). "Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications". Nat Clin Pract Endocrinol Metab 2 (6): 318–327. doi:10.1038/ncpendmet0196. PMID 16932309.
- ^ GreGreen ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, Chua SC Jr, Leibel RL, Weissenbach J, Friedman JM (August 1995). "The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7". Genome Res. 5 (1): 5–12. doi:10.1101/gr.5.1.5. PMID 8717050.
- ^ Ingalls AM, Dickie MM, Snell GD (December 1950). "Obese, a new mutation in the house mouse". J. Hered. 41 (12): 317–8. PMID 14824537. http://jhered.oxfordjournals.org/cgi/reprint/41/12/317.
- ^ Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (December 1994). "Positional cloning of the mouse obese gene and its human homologue". Nature 372 (6505): 425–432. doi:10.1038/372425a0. PMID 7984236.
- ^ a b Margetic S, Gazzola C, Pegg GG, Hill RA (2002). "Leptin: a review of its peripheral actions and interactions". Int. J. Obes. Relat. Metab. Disord. 26 (11): 1407–1433. doi:10.1038/sj.ijo.0802142. PMID 12439643.
- ^ Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ (August 1998). "The stomach is a source of leptin". Nature 394 (6695): 790–793. doi:10.1038/29547. PMID 9723619.
- ^ Studies include:
- Dubuc G, Phinney S, Stern J, Havel P (1998). "Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women". Metab. Clin. Exp. 47 (4): 429–34. doi:10.1016/S0026-0495(98)90055-5. PMID 9550541.
- Pratley R, Nicolson M, Bogardus C, Ravussin E (1997). "Plasma leptin responses to fasting in Pima Indians". Am. J. Physiol. 273 (3 Pt 1): E644–9. PMID 9316457.
- Weigle D, Duell P, Connor W, Steiner R, Soules M, Kuijper J (1997). "Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels". J. Clin. Endocrinol. Metab. 82 (2): 561–565. doi:10.1210/jc.82.2.561. PMID 9024254.
- ^ Chin-Chance C, Polonsky K, Schoeller D (2000). "Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake". J. Clin. Endocrinol. Metab. 85 (8): 2685–2691. doi:10.1210/jc.85.8.2685. PMID 10946866.
- ^ Keim N, Stern J, Havel P (1998). "Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women". Am. J. Clin. Nutr. 68 (4): 794–801. PMID 9771856.
- ^ Mars M, de Graaf C, de Groot C, van Rossum C, Kok F (2006). "Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet". International journal of obesity (Lond) 30 (1): 122–128. doi:10.1038/sj.ijo.0803070. PMID 16158086.
- ^ Kus I, Sarsilmaz M, Colakoglu N et al. (2004). "Pinealectomy increases and exogenous melatonin decreases leptin production in rat anterior pituitary cells: an immunohistochemical study". Physiological research / Academia Scientiarum Bohemoslovaca 53 (4): 403–8. PMID 15311999.
- ^ Alonso-Vale MI, Andreotti S, Peres SB, Anhê GF, das Neves Borges-Silva C, Neto JC, Lima FB (April 2005). "Melatonin enhances leptin expression by rat adipocytes in the presence of insulin". Am. J. Physiol. Endocrinol. Metab. 288 (4): E805–E812. doi:10.1152/ajpendo.00478.2004. PMID 15572654.
- ^ Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH (March 2010). "Leptin therapy in insulin-deficient type I diabetes". Proc. Natl. Acad. Sci. U.S.A. 107 (11): 4813–4819. doi:10.1073/pnas.0909422107. PMC 2841945. PMID 20194735. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2841945. Lay summary – medicinenet.com.
- ^ Williams KW, Scott MM, Elmquist JK (March 2009). "From observation to experimentation: leptin action in the mediobasal hypothalamus". Am. J. Clin. Nutr. 89 (3): 985S–990S. doi:10.3945/ajcn.2008.26788D. PMC 2667659. PMID 19176744. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2667659.
- ^ a b Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD (May 2008). "Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies". Proc. Natl. Acad. Sci. U.S.A. 105 (20): 7257–7262. doi:10.1073/pnas.0706473105. PMC 2438237. PMID 18458326. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2438237.
- ^ Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA (October 2004). "Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy". J. Clin. Endocrinol. Metab. 89 (10): 4821–4826. doi:10.1210/jc.2004-0376. PMID 15472169.
- ^ Friedman JM, Halaas JL (October 1998). "Leptin and the regulation of body weight in mammals". Nature 395 (6704): 763–770. doi:10.1038/27376. PMID 9796811.
- ^ Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M (October 1999). "Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial". JAMA 282 (16): 1568–1575. doi:10.1001/jama.282.16.1568. PMID 10546697.
- ^ Lo KM, Zhang J, Sun Y, Morelli B, Lan Y, Lauder S, Brunkhorst B, Webster G, Hallakou-Bozec S, Doaré L, Gillies SD (January 2005). "Engineering a pharmacologically superior form of leptin for the treatment of obesity". Protein Eng. Des. Sel. 18 (1): 1–10. doi:10.1093/protein/gzh102. PMID 15790575.
- ^ Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z. (2007). "Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis". Arterioscler Thromb Vasc Biol. 27 (12): 2691–2698. doi:10.1161/ATVBAHA.107.149567. PMID 17690315.
- ^ Zhang W, Telemaque S, Augustyniak R, Anderson P, Thomas G, An J, Wang Z, Newgard C, Victor R. (2010). "Adenovirus-mediated leptin expression normalises hypertension associated with diet-induced obesity". J Neuroendocrinol. 22 (3): 175–180. doi:10.1111/j.1365-2826.2010.01953.x. PMID 20059648.
- ^ Knight W, Seth R, Boron J, Overton J. (2009). "Short-term physiological hyperleptinemia decreases arterial blood pressure". Regul Pept. 154 (1–3): 60–68. doi:10.1016/j.regpep.2009.02.001. PMID 19323984.
- ^ John S. Torday, Virender K. Rehan (2006). "Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene regulatory network". Pediatr. Res. 60 (4): 382–388. doi:10.1203/01.pdr.0000238326.42590.03. PMID 16940239. — published online before print as DOI 10.1203/01.pdr.0000238326.42590.03
- ^ Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Sioutopoulou D, Vamvakopoulos N (April 2005). "Estradiol and leptin as conditional prognostic IVF markers". Reproduction 129 (4): 531–534. doi:10.1530/rep.1.00567. PMID 15798029.
- ^ Zhao J, Townsend KL, Schulz LC, Kunz TH, Li C, Widmaier EP (2004). "Leptin receptor expression increases in placenta, but not hypothalamus, during gestation in Mus musculus and Myotis lucifugus". Placenta 25 (8–9): 712–722. doi:10.1016/j.placenta.2004.01.017. PMID 15450389.
- ^ Moynihan AT, Hehir MP, Glavey SV, Smith TJ, Morrison JJ (2006). "Inhibitory effect of leptin on human uterine contractility in vitro". Am. J. Obstet. Gynecol. 195 (2): 504–509. doi:10.1016/j.ajog.2006.01.106. PMID 16647683.
- ^ Aka N, Atalay S, Sayharman S, Kiliç D, Köse G, Küçüközkan T (2006). "Leptin and leptin receptor levels in pregnant women with hyperemesis gravidarum". The Australian & New Zealand journal of obstetrics & gynaecology 46 (4): 274–277. doi:10.1111/j.1479-828X.2006.00590.x. PMID 16866785.
- ^ Cervero A, Domínguez F, Horcajadas JA, Quiñonero A, Pellicer A, Simón C (2006). "The role of the leptin in reproduction". Curr. Opin. Obstet. Gynecol. 18 (3): 297–303. doi:10.1097/01.gco.0000193004.35287.89. PMID 16735830.
- ^ Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP (2007). "Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice". Peptides 28 (5): 1012–1019. doi:10.1016/j.peptides.2007.02.001. PMC 1986832. PMID 17346852. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1986832.
- ^ Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (January 2000). "Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass". Cell 100 (2): 197–207. doi:10.1016/S0092-8674(00)81558-5. PMID 10660043.
- ^ Hamrick MW, Ferrari SL (July 2008). "Leptin and the sympathetic connection of fat to bone". Osteoporos Int 19 (7): 905–912. doi:10.1007/s00198-007-0487-9. PMID 17924050.
- ^ a b Allison SJ, Herzog H (2006). "NPY and bone". EXS (95): 171–82. PMID 16383006.
- ^ Gordeladze JO, Reseland JE (March 2003). "A unified model for the action of leptin on bone turnover". J. Cell. Biochem. 88 (4): 706–712. doi:10.1002/jcb.10385. PMID 12577304.
- ^ Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (November 2002). "Leptin regulates bone formation via the sympathetic nervous system". Cell 111 (3): 305–317. doi:10.1016/S0092-8674(02)01049-8. PMID 12419242.
- ^ a b c Pilon B. "Leptin and Inflammation | Inflammation Theory". Press75.com. http://www.inflammationtheory.com/leptin-and-inflammation/. Retrieved 2011-04-19.
- ^ Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS (May 2003). "The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men". J. Clin. Invest. 111 (9): 1409–1421. doi:10.1172/JCI17490. PMC 154448. PMID 12727933. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=154448.
- ^ Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA (February 2011). "Leptin, Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome". Med. Sci. Monit. 17 (3): CR159–64. PMID 21358603.
- ^ Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH (August 2003). "Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment". Eur. Respir. J. 22 (2): 251–257. doi:10.1183/09031936.03.00010103. PMID 12952256.
- ^ Seaborg, E (2007). "Growing evidence links too little sleep to obesity and diabetes". Endocrine News: 14–15.
- ^ Knutson KL, Spiegel K, Penev P, Van Cauter E (June 2007). "The metabolic consequences of sleep deprivation". Sleep Med Rev 11 (3): 163–178. doi:10.1016/j.smrv.2007.01.002. PMC 1991337. PMID 17442599. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1991337.
- ^ Otsuka R, Yatsuya H, Tamakoshi K, Matsushita K, Wada K, Toyoshima H (October 2006). "Perceived psychological stress and serum leptin concentrations in Japanese men". Obesity (Silver Spring) 14 (10): 1832–1838. doi:10.1038/oby.2006.211. PMID 17062814.
- ^ Ahima RS, Flier JS (2000). "Leptin". Annu. Rev. Physiol. 62: 413–437. doi:10.1146/annurev.physiol.62.1.413. PMID 10845097.
- ^ de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E (July 2010). "Effects of resistance training on cytokines". Int J Sports Med 31 (7): 441–450. doi:10.1055/s-0030-1251994. PMID 20432196.
- ^ Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (August 1998). "Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression". Nature 394 (6696): 897–901. doi:10.1038/29795. PMID 9732873.
- ^ a b Fantuzzi G, Faggioni R (October 2000). "Leptin in the regulation of immunity, inflammation, and hematopoiesis". J. Leukoc. Biol. 68 (4): 437–46. PMID 11037963.
- ^ Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP (March 2001). "Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action?". J. Leukoc. Biol. 69 (3): 414–8. PMID 11261788.
- ^ Madej T, Boguski MS, Bryant SH (October 1995). "Threading analysis suggests that the obese gene product may be a helical cytokine". FEBS Lett. 373 (1): 13–18. doi:10.1016/0014-5793(95)00977-H. PMID 7589424.
- ^ Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS (September 1997). "Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress". Endocrinology 138 (9): 3859–3863. doi:10.1210/en.138.9.3859. PMID 9275075.
- ^ Mabuchi T, Yatsuya H, Tamakoshi K, Otsuka R, Nagasawa N, Zhang H, Murata C, Wada K, Ishikawa M, Hori Y, Kondo T, Hashimoto S, Toyoshima H (2005). "Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women". Diabetes Metab. Res. Rev. 21 (5): 441–447. doi:10.1002/dmrr.540. PMID 15724240.
- ^ Hamilton BS, Paglia D, Kwan AY, Deitel M (September 1995). "Increased obese mRNA expression in omental fat cells from massively obese humans". Nat. Med. 1 (9): 953–956. doi:10.1038/nm0995-953. PMID 7585224.
- ^ Perrier S, Caldefie-Chezet F, Vasson MP. (2009) L-1 family in breast cancer: potential interplay with leptin and other adipocytokines. FEBS Lett. 583(2):259-65>http://www.ncbi.nlm.nih.gov/pubmed/19111549
- ^ Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H (October 1996). "Insulin and cortisol promote leptin production in cultured human fat cells". Diabetes 45 (10): 1435–1438. doi:10.2337/diabetes.45.10.1435. PMID 8826983.
- ^ LaPensee CR, Hugo ER, Ben-Jonathan N (November 2008). "Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways". Endocrinology 149 (11): 5415–5422. doi:10.1210/en.2008-0549. PMC 2584585. PMID 18617614. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2584585.
- ^ Worm D, Vinten J, Vaag A, Henriksen JE, Beck-Nielsen H (September 2000). "The nicotinic acid analogue acipimox increases plasma leptin and decreases free fatty acids in type 2 diabetic patients". Eur. J. Endocrinol. 143 (3): 389–395. doi:10.1530/eje.0.1430389. PMID 11022182.
- ^ Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL (February 1996). "Serum immunoreactive-leptin concentrations in normal-weight and obese humans". N. Engl. J. Med. 334 (5): 292–295. doi:10.1056/NEJM199602013340503. PMID 8532024.
- ^ Tommy Jönsson et al. (2005). "Agrarian diet and diseases of affluence--do evolutionary novel dietary lectins cause leptin resistance?". BMC Endocrine Disorders 5: 10. doi:10.1186/1472-6823-5-10. PMC 1326203. PMID 16336696. http://www.biomedcentral.com/1472-6823/5/10.
- ^ Alexandra Shapiro et al. (2008). "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding". American journal of physiology. Regulatory, integrative and comparative physiology 295 (5): R1370–R1375. doi:10.1152/ajpregu.00195.2008. PMC 2584858. PMID 18703413. http://ajpregu.physiology.org/content/295/5/R1370.full.
- ^ Ashwini Oswal and Giles Yeo (2010). "Leptin and the Control of Body Weight: A Review of Its Diverse Central Targets, Signaling Mechanisms, and Role in the Pathogenesis of Obesity". Obesity 18 (2): 221–229. doi:10.1038/oby.2009.228. PMID 19644451. http://www.nature.com/oby/journal/v18/n2/full/oby2009228a.html.
- ^ The effects of high fat diets on the blood–brain barrier transport of leptin: Failure or adaptation?. http://www.leptinresearch.org/pdf/rsh_high_fat_diets_and_leptin.pdf.
- ^ Myers MG, Cowley MA, Münzberg H (2008). "Mechanisms of leptin action and leptin resistance". Annu. Rev. Physiol. 70: 537–556. doi:10.1146/annurev.physiol.70.113006.100707. PMID 17937601.
- ^ Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L (December 2001). "Overfeeding rapidly induces leptin and insulin resistance". Diabetes 50 (12): 2786–2791. doi:10.2337/diabetes.50.12.2786. PMID 11723062.
- ^ Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA (March 2007). "Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons". Cell Metab. 5 (3): 181–194. doi:10.1016/j.cmet.2007.02.004. PMID 17339026.
- ^ Obici, S; Rossetti, L (2003). "Minireview: Nutrient Sensing and the Regulation of Insulin Action and Energy Balance". Endocrinology 144 (12): 5172–5178. doi:10.1210/en.2003-0999. PMID 12970158. http://endo.endojournals.org/cgi/content/full/144/12/5172.
- ^ "Fructose Sets Table For Weight Gain Without Warning". Science News. Science Daily. 2008-10-19. http://www.sciencedaily.com/releases/2008/10/081016074701.htm. Retrieved 2008-11-15.
- ^ Vasselli JR (November 2008). "Fructose-induced leptin resistance: discovery of an unsuspected form of the phenomenon and its significance. Focus on "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding," by Shapiro et al". Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 (5): R1365–R1369. doi:10.1152/ajpregu.90674.2008. PMID 18784330.
- ^ Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ (November 2008). "Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding". Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 (5): R1370–R1375. doi:10.1152/ajpregu.00195.2008. PMC 2584858. PMID 18703413. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2584858.
- ^ Wang MY, Zhou YT, Newgard CB, Unger RH (August 1996). "A novel leptin receptor isoform in rat". FEBS Lett. 392 (2): 87–90. doi:10.1016/0014-5793(96)00790-9. PMID 8772180.
- ^ Malendowicz W, Rucinski M, Macchi C, Spinazzi R, Ziolkowska A, Nussdorfer GG, Kwias Z (October 2006). "Leptin and leptin receptors in the prostate and seminal vesicles of the adult rat". Int. J. Mol. Med. 18 (4): 615–8. PMID 16964413. http://www.spandidos-publications.com/ijmm/article.jsp?article_id=ijmm_18_4_615.
- ^ "LepRb antibody (commercial site)". http://www.neuromics.com/ittrium/visit?path=A1x66x1y1x9fx1y1x246x1y1x372x1x82y1x35d4x1x7f.
- ^ "Amylin to Present Data Showing Investigational Metreleptin Treatment Led to Long-Term Improvements in Diabetes and Lipid Control in Patients with Lipodystrophy". Press Release. Amylin Pharmaceuticals. 2011-04-15. http://investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1550945&highlight. Retrieved 2011-10-27.
- ^ "JDRF & Amylin Partner to Investigate Therapy to Improve Blood Glucose Control". Press Release. JDRF. http://www.jdrf.org/index.cfm?page_id=114682.
Further reading
- Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP (March 2002). "Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation". Am. J. Physiol. Lung Cell Mol. Physiol. 282 (3): L405–10. PMC 2942763. PMID 11839533. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2942763.
- Torday JS, Rehan VK (July 2002). "Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin". Am. J. Physiol. Lung Cell Mol. Physiol. 283 (1): L130–5. doi:10.1152/ajplung.00380.2001 (inactive 2010-08-06). PMID 12060569.
- Dubey L, Hesong Z (2006). "Role of leptin in atherogenesis". Exp Clin Cardiol 11 (4): 269–75. PMC 2274849. PMID 18651016. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2274849.
- Friedman JM, Halaas JL (1998). "Leptin and the regulation of body weight in mammals". Nature 395 (6704): 763–770. doi:10.1038/27376. PMID 9796811.
- Prolo P, Wong ML, Licinio J (1999). "Leptin". Int. J. Biochem. Cell Biol. 30 (12): 1285–1290. doi:10.1016/S1357-2725(98)00094-6. PMID 9924798.
- Heshka JT, Jones PJ (2001). "A role for dietary fat in leptin receptor, OB-Rb, function". Life Sci. 69 (9): 987–1003. doi:10.1016/S0024-3205(01)01201-2. PMID 11508653.
- Janeckova R (2002). "The role of leptin in human physiology and pathophysiology". Physiological research / Academia Scientiarum Bohemoslovaca 50 (5): 443–59. PMID 11702849.
- Lee DW, Leinung MC, Rozhavskaya-Arena M, Grasso P (2002). "Leptin and the treatment of obesity: its current status". Eur. J. Pharmacol. 440 (2–3): 129–139. doi:10.1016/S0014-2999(02)01424-3. PMID 12007531.
- Al-Daghri N, Bartlett WA, Jones AF, Kumar S (2002). "Role of leptin in glucose metabolism in type 2 diabetes". Diabetes, obesity & metabolism 4 (3): 147–155. doi:10.1046/j.1463-1326.2002.00194.x. PMID 12047393.
- Sabath Silva EF (2002). "[Leptin]". Rev. Invest. Clin. 54 (2): 161–5. PMID 12053815.
- Thomas T, Burguera B (2003). "Is leptin the link between fat and bone mass?". J. Bone Miner. Res. 17 (9): 1563–1569. doi:10.1359/jbmr.2002.17.9.1563. PMID 12211425.
- Kraemer RR, Chu H, Castracane VD (2002). "Leptin and exercise". Exp. Biol. Med. (Maywood) 227 (9): 701–8. PMID 12324651.
- Waelput W, Brouckaert P, Broekaert D, Tavernier J (2003). "A role for leptin in the systemic inflammatory response syndrome (SIRS) and in immune response". Current drug targets. Inflammation and allergy 1 (3): 277–289. doi:10.2174/1568010023344634. PMID 14561193.
- Stenvinkel P, Pecoits-Filho R, Lindholm B (2004). "Leptin, ghrelin, and proinflammatory cytokines: compounds with nutritional impact in chronic kidney disease?". Advances in renal replacement therapy 10 (4): 332–345. doi:10.1053/j.arrt.2003.08.009. PMID 14681862.
- Cohen P, Ntambi JM, Friedman JM (2004). "Stearoyl-CoA desaturase-1 and the metabolic syndrome". Curr. Drug Targets Immune Endocr. Metabol. Disord. 3 (4): 271–280. doi:10.2174/1568008033340117. PMID 14683458.
- Sahu A (2004). "Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance". Frontiers in neuroendocrinology 24 (4): 225–253. doi:10.1016/j.yfrne.2003.10.001. PMID 14726256.
- Elefteriou F, Karsenty G (2004). "[Bone mass regulation by leptin: a hypothalamic control of bone formation]". Pathol. Biol. 52 (3): 148–153. doi:10.1016/j.patbio.2003.05.006. PMID 15063934.
- Blüher S, Mantzoros CS (2004). "The role of leptin in regulating neuroendocrine function in humans". J. Nutr. 134 (9): 2469S–2474S. PMID 15333744.
- Farooqi S, O'Rahilly S (2007). "Genetics of obesity in humans". Endocr. Rev. 27 (7): 710–18. doi:10.1210/er.2006-0040. PMID 17122358.
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL (February 1996). "Serum immunoreactive-leptin concentrations in normal-weight and obese humans". N. Engl. J. Med. 334 (5): 292–5. doi:10.1056/NEJM199602013340503. PMID 8532024.
External links
- Leptin in a bulletin by the Howard Hughes Medical Institute (HHMI)
- Leptin: Your brain, appetite and obesity by the British Society of Neuroendocrinology
- Leptin/ghrelin and their role in obesity from hungerhormones.com, a weight control website
- Leptin by Colorado State University
- Leptin at 3Dchem.com, description and structure diagrams
Endocrine system: hormones (Peptide hormones · Steroid hormones) Endocrine
glandsTestis: testosterone · AMH · inhibin
Ovary: estradiol · progesterone · activin and inhibin · relaxin (pregnancy)
Placenta: hCG · HPL · estrogen · progesteroneIslet-Acinar
AxisNon-end.
glandsThymus: Thymosin (Thymosin α1, Thymosin beta) · Thymopoietin · Thymulin
Digestive system: Stomach: gastrin · ghrelin · Duodenum: CCK · GIP · secretin · motilin · VIP · Ileum: enteroglucagon · peptide YY · Liver/other: Insulin-like growth factor (IGF-1, IGF-2)
Adipose tissue: leptin · adiponectin · resistin
Kidney: JGA (renin) · peritubular cells (EPO) · calcitriol · prostaglandin
Heart: Natriuretic peptide (ANP, BNP)Categories:- Human proteins
- Peptide hormones
Wikimedia Foundation. 2010.