- Nitric oxide
-
Not to be confused with nitrous oxide or nitrogen oxides.For other uses, see NO (disambiguation).
Nitric oxide 

 Nitric oxideSystematic nameOxidonitrogen(•)[1] (additive)Other namesNitrogen monoxide
Nitric oxideSystematic nameOxidonitrogen(•)[1] (additive)Other namesNitrogen monoxide
Nitrogen(II) oxideIdentifiers CAS number 10102-43-9 
PubChem 145068 ChemSpider 127983 
UNII 31C4KY9ESH 
EC number 233-271-0 UN number 1660 DrugBank DB00435 KEGG D00074 
ChEBI CHEBI:16480 
ChEMBL CHEMBL1200689 
RTECS number QX0525000 ATC code R07 Gmelin Reference 451 3DMet B00122 Jmol-3D images Image 1 - [N]=O
Properties Molecular formula NO Molar mass 30.01 g mol−1 Exact mass 29.997988627 g mol−1 Appearance Colourless gas Density 1.3402 g dm−3 Melting point −164 °C, 109 K, -263 °F
Boiling point −152 °C, 121 K, -242 °F
Solubility in water 74 cm3 dm−3 Refractive index (nD) 1.0002697 Structure Molecular shape linear (point group C∞v) Thermochemistry Std enthalpy of
formation ΔfHo29890.29 kJ mol−1 Standard molar
entropy So298210.76 J K−1 mol−1 Pharmacology Bioavailability good Routes of
administrationInhalation Metabolism via pulmonary capillary bed Elimination
half-life2–6 seconds Hazards MSDS External MSDS EU classification  O
O  T
TR-phrases R8, R23, R34, R44 S-phrases (S1), S17, S23, S36/37/39, S45 NFPA 704 Related compounds Related nitrogen oxides Dinitrogen pentoxide
Dinitrogen tetroxide
Dinitrogen trioxide
Nitrogen dioxide
Nitrous oxide oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical[2] and is an important intermediate in the chemical industry. Nitric oxide is a by-product of combustion of substances in the air, as in automobile engines, fossil fuel power plants, or lightning.
In mammals including humans, NO is an important cellular signaling molecule involved in many physiological and pathological processes.[3] Low levels of NO production are important in protecting an organ such as the liver from ischemic damage. Chronic expression of NO is associated with various carcinomas and inflammatory conditions including juvenile diabetes, multiple sclerosis, arthritis and ulcerative colitis.[citation needed]
Nitric oxide should not be confused with nitrous oxide (N2O), an anaesthetic and greenhouse gas, or with nitrogen dioxide (NO2), a brown toxic gas and a major air pollutant. However, nitric oxide is rapidly oxidised in air to nitrogen dioxide. Humphrey Davy discovered this to his discomfort, when he inhaled the gas early in his career.
Despite being a simple molecule, NO is a fundamental component in the fields of neuroscience, physiology, and immunology, and was proclaimed “Molecule of the Year” in 1992.[4]
Contents
Reactions
- When exposed to oxygen, NO is converted into nitrogen dioxide.
-
- 2 NO + O2 → 2 NO2
- This conversion has been speculated as occurring via the ONOONO intermediate. In water, NO reacts with oxygen and water to form HNO2 or nitrous acid. The reaction is thought to proceed via the following stoichiometry:
- 4 NO + O2 + 2 H2O → 4 HNO2
- NO will react with fluorine, chlorine, and bromine to form the XNO species, known as the nitrosyl halides, such as nitrosyl chloride. Nitrosyl iodide can form but is an extremely short-lived species and tends to reform I2.
-
- 2 NO + Cl2 → 2 NOCl
- Nitroxyl (HNO) is the reduced form of nitric oxide.
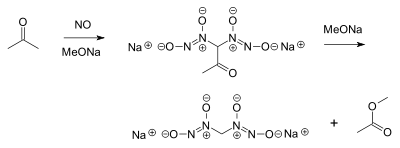
- Nitric oxide reacts with acetone and an alkoxide to a diazeniumdiolate or nitrosohydroxylamine and methyl acetate:[5]
- This is a very old reaction (1898) but of interest today in NO prodrug research. Nitric oxide can also react directly with sodium methoxide, forming sodium formate and nitrous oxide.[6]
Preparation
Commercially, NO is produced by the oxidation of ammonia at 750 °C to 900 °C (normally at 850 °C) with platinum as catalyst:
- 4 NH3 + 5 O2 → 4 NO + 6 H2O
The uncatalyzed endothermic reaction of O2 and N2, which is performed at high temperature (>2000 °C) by lightning has not been developed into a practical commercial synthesis (see Birkeland–Eyde process):
- N2 + O2 → 2 NO
In the laboratory, nitric oxide is conveniently generated by reduction of nitric acid with copper:
- 8 HNO3 + 3 Cu → 3 Cu(NO3)2 + 4 H2O + 2 NO
or by the reduction of nitrous acid in the form of sodium nitrite or potassium nitrite:
- 2 NaNO2 + 2 NaI + 2 H2SO4 → I2 + 4 NaHSO4 + 2 NO
- 2 NaNO2 + 2 FeSO4 + 3 H2SO4 → Fe2(SO4)3 + 2 NaHSO4 + 2 H2O + 2 NO
- 3 KNO2 (l) + KNO3 (l) + Cr2O3(s) → 2 K2CrO4(s) + 4 NO (g)
The iron(II) sulfate route is simple and has been used in undergraduate laboratory experiments.
So-called NONOate compounds are also used for NO generation.
Coordination chemistry
Main article: Metal nitrosylNO reacts with all transition metals to give complexes called metal nitrosyls. The most common bonding mode of NO is the terminal linear type (M-NO). The angle of the M-N-O group varies from 160° to 180° but is still termed "linear". In this case, the NO group is considered a 3-electron donor under the covalent (neutral) method of electron counting, or a 2-electron donor under the ionic method.[7] In the case of a bent M-N-O conformation, the NO group can be considered a one-electron donor using neutral counting, or a 2-electron donor using ionic counting.[8] One can view such complexes as derived from NO+, which is isoelectronic with CO.
Nitric oxide can serve as a one-electron pseudohalide. In such complexes, the M-N-O group is characterized by an angle between 120° and 140°.
The NO group can also bridge between metal centers through the nitrogen atom in a variety of geometries.
Measurement of nitric oxide concentration
 Nitric oxide (white) in conifer cells, visualized using DAF-2 DA (diaminofluorescein diacetate)
Nitric oxide (white) in conifer cells, visualized using DAF-2 DA (diaminofluorescein diacetate)
Nitric oxide concentration can be determined using a simple chemiluminescent reaction involving ozone:[9] A sample containing nitric oxide is mixed with a large quantity of ozone. The nitric oxide reacts with the ozone to produce oxygen and nitrogen dioxide. This reaction also produces light (chemiluminescence), which can be measured with a photodetector. The amount of light produced is proportional to the amount of nitric oxide in the sample.
- NO + O3 → NO2 + O2 + light
Other methods of testing include electroanalysis (amperometric approach), where NO reacts with an electrode to induce a current or voltage change. The detection of NO radicals in biological tissues is particularly difficult due to the short lifetime and concentration of these radicals in tissues. One of the few practical methods is spin trapping of nitric oxide with iron-dithiocarbamate complexes and subsequent detection of the mono-nitrosyl-iron complex with electron paramagnetic resonance (EPR).[10][11]
A group of fluorescent dye indicators that are also available in acetylated form for intracellular measurements exist. The most common compound is 4,5-diaminofluorescein (DAF-2).[4]
Production
From a thermodynamic perspective, NO is unstable with respect to O2 and N2, although this conversion is very slow at ambient temperatures in the absence of a catalyst. Because the heat of formation of NO is endothermic, its synthesis from molecular nitrogen and oxygen requires elevated temperatures above 1000 °C. A major natural source is lightning. The use of internal combustion engines has drastically increased the presence of nitric oxide in the environment. One purpose of catalytic converters in cars is to minimize NO emission by catalytic reversion to O2 and N2.
Environmental effects
Nitric oxide in the air may convert to nitric acid, which has been implicated in acid rain. Furthermore, both NO and NO2 participate in ozone layer depletion. Nitric oxide is a small highly diffusible gas and a ubiquitous bioactive molecule.
Technical applications
Although NO has relatively few direct uses, it is produced on a massive scale as an intermediate in the Ostwald process for the synthesis of nitric acid from ammonia. In 2005, the US alone produced 6 million metric tons of nitric acid.[12] It finds use in the semiconductor industry for various processes. In one of its applications it is used along with nitrous oxide to form oxynitride gates in CMOS devices.
Miscellaneous applications
Nitric oxide can be used for detecting surface radicals on polymers. Quenching of surface radicals with nitric oxide results in incorporation of nitrogen, which can be quantified by means of X-ray photoelectron spectroscopy.
Biological functions
Main article: Biological functions of nitric oxideNO is one of the few gaseous signaling molecules known and is additionally exceptional due to the fact that it is a radical gas. It is a key vertebrate biological messenger, playing a role in a variety of biological processes. Nitric oxide, known as the 'endothelium-derived relaxing factor', or 'EDRF', is biosynthesized endogenously from L-arginine, oxygen and NADPH by various nitric oxide synthase (NOS) enzymes. Reduction of inorganic nitrate may also serve to make nitric oxide. The endothelium (inner lining) of blood vessels uses nitric oxide to signal the surrounding smooth muscle to relax, thus resulting in vasodilation and increasing blood flow. Nitric oxide is highly reactive (having a lifetime of a few seconds), yet diffuses freely across membranes. These attributes make nitric oxide ideal for a transient paracrine (between adjacent cells) and autocrine (within a single cell) signaling molecule.[13] The production of nitric oxide is elevated in populations living at high altitudes, which helps these people avoid hypoxia by aiding in pulmonary vasculature vasodilation. Effects include vasodilatation, neurotransmission (see gasotransmitters), modulation of the hair cycle,[14] production of reactive nitrogen intermediates and penile erections (through its ability to vasodilate). Nitroglycerin and amyl nitrite serve as vasodilators because they are converted to nitric oxide in the body. The vasodialating antihypertensive drug minoxidil contains an NO moity and may act as an NO agonist. Similarly, Sildenafil citrate, popularly known by the trade name Viagra, stimulates erections primarily by enhancing signaling through the nitric oxide pathway in the penis.
Nitric oxide (NO) contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to the endothelium. Humans with atherosclerosis, diabetes, or hypertension often show impaired NO pathways.[15] A high salt intake was demonstrated to attenuate NO production, although bioavailability remains unregulated.[16]
Nitric oxide is also generated by phagocytes (monocytes, macrophages, and neutrophils) as part of the human immune response. Phagocytes are armed with inducible nitric oxide synthase (iNOS), which is activated by interferon-gamma (IFN-γ) as a single signal or by tumor necrosis factor (TNF) along with a second signal.[17] On the other hand, transforming growth factor-beta (TGF-β) provides a strong inhibitory signal to iNOS, whereas interleukin-4 (IL-4) and IL-10 provide weak inhibitory signals. In this way the immune system may regulate the armamentarium of phagocytes that play a role in inflammation and immune responses. Nitric oxide secreted as an immune response is as free radicals and is toxic to bacteria; the mechanism for this includes DNA damage[18][19][20] and degradation of iron sulfur centers into iron ions and iron-nitrosyl compounds.[21] In response, however, many bacterial pathogens have evolved mechanisms for nitric oxide resistance.[22] Because nitric oxide might serve as an inflammometer in conditions like asthma, there has been increasing interest in the use of exhaled nitric oxide as a breath test in diseases with airway inflammation. Reduced levels of exhaled NO have been associated with exposure to air pollution.[23]
Nitric oxide can contribute to reperfusion injury when an excessive amount produced during reperfusion (following a period of ischemia) reacts with superoxide to produce the damaging oxidant peroxynitrite. In contrast, inhaled nitric oxide has been shown to help survival and recovery from paraquat poisoning, which produces lung tissue–damaging superoxide and hinders NOS metabolism.
In plants, nitric oxide can be produced by any of four routes: (i) L-arginine-dependent nitric oxide synthase,[24][25][26] (although the existence of animal NOS homologs in plants is debated),[27] (ii) by plasma membrane-bound nitrate reductase, (iii) by mitochondrial electron transport chain, or (iv) by non-enzymatic reactions. It is a signaling molecule, acts mainly against oxidative stress and also plays a role in plant pathogen interactions. Treating cut flowers and other plants with nitric oxide has been shown to lengthen the time before wilting.[28]
An important biological reaction of nitric oxide is S-nitrosylation, the conversion of thiol groups, including cysteine residues in proteins, to form S-nitrosothiols (RSNOs). S-Nitrosylation is a mechanism for dynamic, post-translational regulation of most or all major classes of protein.
Mechanism of action
There are several mechanisms by which NO has been demonstrated to affect the biology of living cells. These include oxidation of iron-containing proteins such as ribonucleotide reductase and aconitase, activation of the soluble guanylate cyclase, ADP ribosylation of proteins, protein sulfhydryl group nitrosylation, and iron regulatory factor activation.[29] NO has been demonstrated to activate NF-κB in peripheral blood mononuclear cells, an important transcription factor in iNOS gene expression in response to inflammation.[30] It was found that NO acts through the stimulation of the soluble guanylate cyclase, which is a heterodimeric enzyme with subsequent formation of cyclic GMP. Cyclic GMP activates protein kinase G, which causes phosphorylation of myosin light chain phosphatase, and therefore inactivation of myosin light-chain kinase, and leads ultimately to the dephosphorylation of the myosin light chain, causing smooth muscle relaxation.[31]
Medical use
Neonatal and pediatric use
Nitric oxide/oxygen blends are used in critical care to promote capillary and pulmonary dilation to treat primary pulmonary hypertension in neonatal patients[32][33] post-meconium aspiration and related to birth defects. These are often a last-resort gas mixture before the use of extracorporeal membrane oxygenation (ECMO). Nitric oxide therapy has the potential to significantly increase the quality of life and, in some cases, save the lives of infants at risk for pulmonary vascular disease.[34]
Pharmacology
Nitric oxide is considered an antianginal drug: it causes vasodilation, which can help with ischemic pain, known as angina, by decreasing the cardiac workload. By dilating (expanding) the veins, nitric oxide drugs lower arterial pressure and left ventricular filling pressure.[35] This vasodilation does not decrease the volume of blood the heart pumps, but rather it decreases the force the heart muscle must exert to pump the same volume of blood. Nitroglycerin pills, taken sublingually (under the tongue), are used to prevent or treat acute chest pain. The nitroglycerin reacts with a sulfhydryl group (–SH) to produce nitric oxide, which eases the pain by causing vasodilation. Recent evidence suggests that nitrates may be beneficial for treatment of angina due to reduced myocardial oxygen consumption both by decreasing preload and afterload and by some direct vasodilation of coronary vessels.[35]
Mainly for its utility as a vasodilator, NO has entered the fitness industry as an array of supplements geared towards accelerated muscle growth and prolonged stamina during endurance activities.
References
- ^ "Nitric Oxide (CHEBI:16480)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=16480.
- ^ Principles and Applications of ESR Spectroscopy , Anders Lund,Masaru Shiotani,Shigetaka Shimada 2010
- ^ Hou, YC; Janczuk, A; Wang, PG (1999). "Current trends in the development of nitric oxide donors.". Current pharmaceutical design 5 (6): 417–41. PMID 10390607.
- ^ a b Elizabeth Culotta and Daniel E. Koshland Jr (1992). "NO news is good news. (nitric oxide; includes information about other significant advances & discoveries of 1992) (Molecule of the Year)". Science 258 (5090): 1862–1864. doi:10.1126/science.1361684. PMID 1361684.
- ^ Traube, Wilhelm (1898). "Ueber Synthesen stickstoffhaltiger Verbindungen mit Hülfe des Stickoxyds". Justus Liebig's Annalen der Chemie 300: 81. doi:10.1002/jlac.18983000108.
- ^ Derosa, Frank; Keefer, Larry K.; Hrabie, Joseph A. (2008). "Nitric Oxide Reacts with Methoxide". The Journal of Organic Chemistry 73 (3): 1139–42. doi:10.1021/jo7020423. PMID 18184006.
- ^ Robert H. Crabtree: "The Organometallic Chemistry of the Transition Metals", John Wiley and Sons, 2005, ISBN 0471662569, p. 32.
- ^ Robert H. Crabtree: "The Organometallic Chemistry of the Transition Metals", John Wiley and Sons, 2005, ISBN 0471662569, pp. 96–98.
- ^ Fontijn, Arthur.; Sabadell, Alberto J.; Ronco, Richard J. (1970). "Homogeneous chemiluminescent measurement of nitric oxide with ozone. Implications for continuous selective monitoring of gaseous air pollutants". Analytical Chemistry 42 (6): 575. doi:10.1021/ac60288a034.
- ^ Vanin, A; Huisman, A; Vanfaassen, E (2002). "Iron dithiocarbamate as spin trap for nitric oxide detection: Pitfalls and successes". Methods in enzymology 359: 27. doi:10.1016/S0076-6879(02)59169-2. PMID 12481557.
- ^ Nagano, T; Yoshimura, T (2002). "Bioimaging of nitric oxide". Chemical reviews 102 (4): 1235–70. doi:10.1021/cr010152s. PMID 11942795.
- ^ “Production: Growth is the Norm” Chemical and Engineering News, July 10, 2006, p. 59.
- ^ Stryer, Lubert (1995). Biochemistry, 4th Edition. W.H. Freeman and Company. pp. 732. ISBN 0-7167-2009-4.
- ^ Alopecia & Free Radical " Redox " Signaling-Nitric Oxide and Superoxide
- ^ Dessy, C.; Ferron, O. (2004). "Pathophysiological Roles of Nitric Oxide: In the Heart and the Coronary Vasculature". Current Medical Chemistry – Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry 3 (3): 207–216. doi:10.2174/1568014043355348.
- ^ Osanai, T; Fujiwara, N; Saitoh, M; Sasaki, S; Tomita, H; Nakamura, M; Osawa, H; Yamabe, H et al. (2002). "Relationship between salt intake, nitric oxide and asymmetric dimethylarginine and its relevance to patients with end-stage renal disease". Blood purification 20 (5): 466–8. doi:10.1159/000063555. PMID 12207094. http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowPDF&ProduktNr=223997&Ausgabe=228460&ArtikelNr=63555.
- ^ Gorczyniski and Stanely, Clinical Immunology. Landes Bioscience; Austin, TX. ISBN 1570596255
- ^ Wink, DA; et.al. (1991). "DNA deaminating ability and genotoxicity of nitric oxide and its progenitors". Science 254 (5034): 1001–3. doi:10.1126/science.1948068. PMID 1948068. About killing of salmonella bacteria.
- ^ Nguyen, T; Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR (1992). "DNA damage and mutation in human cells exposed to nitric oxide in vitro". Proc Natl Acad Sci USA 89 (7): 3030–4. doi:10.1073/pnas.89.7.3030. PMC 48797. PMID 1557408. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=48797. Free text.
- ^ Li, CQ; Pang B, Kiziltepe T, Trudel LJ, Engelward BP, Dedon PC, Wogan GN (2006). "Threshold Effects of Nitric Oxide-Induced Toxicity and Cellular Responses in Wild-Type and p53-Null Human Lymphoblastoid Cells". Chem Res Toxicol 19 (3): 399–406. doi:10.1021/tx050283e. PMC 2570754. PMID 16544944. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2570754. free text.
- ^ Hibbs, JB; Taintor RR, Vavrin Z, Rachlin EM (1988). "Nitric oxide: a cytotoxic activated macrophage effector molecule". Biochem Biophys Res Commun 157 (1): 87–94. doi:10.1016/S0006-291X(88)80015-9. PMID 3196352.
- ^ C. A. Janeway, et al. (2005). Immunobiology: the immune system in health and disease (6th ed.). New York: Garland Science. ISBN 0-8153-4101-6.
- ^ Jacobs, L; Nawrot, Tim S; De Geus, Bas; Meeusen, Romain; Degraeuwe, Bart; Bernard, Alfred; Sughis, Muhammad; Nemery, Benoit et al. (Oct 2010). "Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution". Environmental Health 9 (64): 64. doi:10.1186/1476-069X-9-64. http://www.ehjournal.net/content/9/1/64.
- ^ Corpas, F. J. et al. (2004). "Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants". Plant Physiology 136 (1): 2722–33. doi:10.1104/pp.104.042812. PMC 523336. PMID 15347796. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=523336.
- ^ Corpas, F. J. et al. (2006). "Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development". Planta 224 (2): 246–54. doi:10.1007/s00425-005-0205-9. PMID 16397797.
- ^ Valderrama, R. et al. (2007). "Nitrosative stress in plants". FEBS Lett 581 (3): 453–61. doi:10.1016/j.febslet.2007.01.006. PMID 17240373.
- ^ Corpas et al.; Barroso, Juan B.; Del Rio, Luis A. (2004). "Enzymatic sources of nitric oxide in plant cells – beyond one protein–one function". New Phytologist 162 (2): 246–7. doi:10.1111/j.1469-8137.2004.01058.x.
- ^ Judy Siegel-Itzkovich. Viagra makes flowers stand up straight. Student BMJ, September 1999.
- ^ Shami, PJ; Moore, JO; Gockerman, JP; Hathorn, JW; Misukonis, MA; Weinberg, JB (1995). "Nitric oxide modulation of the growth and differentiation of freshly isolated acute non-lymphocytic leukemia cells". Leukemia research 19 (8): 527–33. doi:10.1016/0145-2126(95)00013-E. PMID 7658698.
- ^ Kaibori M., Sakitani K., Oda M., Kamiyama Y., Masu Y. and Okumura T. (1999). "Immunosuppressant FK506 inhibits inducible nitric oxide synthase gene expression at a step of NF-κB activation in rat hepatocytes". J. Hepatol. 30 (6): 1138–1145. doi:10.1016/S0168-8278(99)80270-0. PMID 10406194. http://www.jhep-elsevier.com/article/S0168-8278(99)80270-0/abstract.
- ^ Surks, HK (2007). "cGMP-dependent protein kinase I and smooth muscle relaxation: a tale of two isoforms". Circulation research 101 (11): 1078–80. doi:10.1161/CIRCRESAHA.107.165779. PMID 18040024.
- ^ Finer NN, Barrington KJ (2006). Finer, Neil. ed. "Nitric oxide for respiratory failure in infants born at or near term". Cochrane Database Syst Rev (4): CD000399. doi:10.1002/14651858.CD000399.pub2. PMID 17054129.
- ^ Chotigeat U, Khorana M, Kanjanapattanakul W (2007). "Inhaled nitric oxide in newborns with severe hypoxic respiratory failure". J Med Assoc Thai 90 (2): 266–71. PMID 17375630.
- ^ Hayward, CS; Kelly, RP; MacDonald, PS (1999). "Inhaled nitric oxide in cardiology practice". Cardiovascular research 43 (3): 628–38. doi:10.1016/S0008-6363(99)00114-5. PMID 10690334.
- ^ a b Abrams, J (1996). "Beneficial actions of nitrates in cardiovascular disease". The American Journal of Cardiology 77 (13): C31. doi:10.1016/S0002-9149(96)00186-5. PMID 8638524.
Further reading
- Butler A. and Nicholson R.; "Life, death and NO." Cambridge 2003. ISBN 978-0-85404-686-7.
- van Faassen, E. E.; Vanin, A. F. (eds); "Radicals for life: The various forms of Nitric Oxide." Elsevier, Amsterdam 2007. ISBN 978-0-444-52236-8.
- Ignarro, L. J. (ed.); "Nitric oxide:biology and pathobiology." Academic Press, San Diego 2000. ISBN 0-12-370420-0.
External links
- International Chemical Safety Card 1311
- National Pollutant Inventory – Oxides of nitrogen Fact Sheet
- 1998 Nobel Prize in Physiology/Medicine for discovery of NO's role in cardiovascular regulation
- Nitric Oxide and its Role in Diabetes, Wound Healing and Peripheral Neuropathy
- Microscale Gas Chemistry: Experiments with Nitrogen Oxides
- Your Brain Boots Up Like a Computer – new insights about the biological role of nitric oxide.
- Assessing The Potential of Nitric Oxide in the Diabetic Foot
- New Discoveries About Nitric Oxide Can Provide Drugs For Schizophrenia
- Nitric Oxide at the Chemical Database
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Carbon monoxide · Hydrogen sulfide · Nitric oxide · Nitrous oxide
Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Oxides
- Inorganic nitrogen compounds
- Neurotransmitters
- Nitrogen metabolism
- Free radicals
Wikimedia Foundation. 2010.