- Fluvoxamine
-
Not to be confused with Fluoxetine.
Fluvoxamine 
Systematic (IUPAC) name (E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one O-2-aminoethyl oxime Clinical data Trade names Luvox AHFS/Drugs.com monograph MedlinePlus a682275 Pregnancy cat. C Legal status Prescription Only (S4) (AU) ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 77% Metabolism Hepatic Half-life 15.6 hours Excretion Renal Identifiers CAS number 54739-18-3 
ATC code N06AB08 PubChem CID 5324346 DrugBank DB00176 ChemSpider 4481878 
UNII O4L1XPO44W 
KEGG D07984 
ChEBI CHEBI:5138 
ChEMBL CHEMBL814 
Chemical data Formula C15H21F3N2O2 Mol. mass 318.335 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Fluvoxamine (brand name Luvox) is an antidepressant which functions as a selective serotonin reuptake inhibitor (SSRI). Fluvoxamine was first approved by the U.S. Food and Drug Administration (FDA) in 1993 for the treatment of obsessive compulsive disorder (OCD).[1] Fluvoxamine CR (controlled release) is approved to treat social anxiety disorder.[2] Fluvoxamine is also prescribed to treat major depressive disorder (MDD) and anxiety disorders, such as panic disorder and post-traumatic stress disorder (PTSD).[3]
Contents
Medical uses
Fluvoxamine's primary use is the treatment of obsessive compulsive disorder (OCD). Fluvoxamine has been found to be useful in the treatment of major depressive disorder (MDD), and anxiety disorders such as panic disorder, social anxiety disorder, post-traumatic stress disorder (PTSD), and obsessive-compulsive spectrum disorders. Fluvoxamine is indicated for children and adolescents with OCD.[4]
Adverse effects
Side effects most commonly observed with fluvoxamine include nausea, vomiting, drowsiness, insomnia, dizziness, nervousness, feeling anxious, dry mouth, abdominal pain, constipation, diarrhea, heart burn, loss of appetite, muscle weakness, pins and needles, abnormal taste, headache, faster heart beat, sweating, weight gain, weight loss or unusual bruising. Other side effects which are observed more frequently in children include abnormal thoughts or behaviour, cough, increased period pain, nose bleeds, increased restlessness, infection and sinusitis.[5] Sexual side effects with fluvoxamine are less pronounced than with other SSRIs.[6]
Pharmacology
Fluvoxamine is a potent and selective serotonin reuptake inhibitor with approximately 100-fold affinity for the serotonin transporter over the norepinephrine transporter. It has negligible affinity for the dopamine transporter or any other receptor, with the sole exception of the σ1 receptor. It behaves as a potent agonist at this receptor and has the highest affinity of any SSRI for doing so. This may contribute to its antidepressant and anxiolytic effects inside the brain. Indeed, other SSRIs which also act as σ1 receptor agonists, such as sertraline and escitalopram (not verified but likely to be), display enhanced antidepressant efficacy.[7] suggesting that it may have particular benefits in the treatment of depressed patients who show features of anxiety/stress and for whom memory impairment is particularly undesirable (such as in depressed elderly patients, and also in treating psychotic depression).[8] In fact, the TCA opipramol, a σ1 receptor agonist without effects on the serotonin, dopamine, or norepinephrine systems, has considerable antidepressant and anxiolytic efficacy in its own right.
Pharmacokinetics
The oral bioavailability of fluvoxamine is 53%. The plasma protein binding is about 80%.[9]
Metabolism
Fluvoxamine is strongly metabolized in the liver, mostly by the processes of oxidative demethylation (producing fluvoxamine acid and its N-acetyl analog) and deamination (producing fluvoxethanol). Only fluvoxamine acid has been shown to have SERT inhibitor activity, roughly 1-2 orders of magnitude less potent than the parent compound.[10]
Radio-labeled administration of a dose of fluvoxamine produced nine identifiable metabolites, constituting 85% of the absorbed dosage (thus 15% of the fluvoxamine remained unchanged). This isolate of metabolites was empirically proven to contain 60% fluvoxamine acid and its N-acetyl analog, and 10% fluvoxethanol, with the other six metabolites making up 30%.[10]
Elimination
Fluvoxamine has the shortest serum half-life of all SSRIs, with a mean of 15.6 hours.[11]
Drug interactions
Fluvoxamine inhibits cytochrome P450 enzyme CYP1A2, which metabolises agomelatine, caffeine, clozapine, haloperidol, phenacetin, tacrine, theophylline, and olanzapine. These substances can cause increased serum levels when administered together with fluvoxamine. Of major concern is the fact that the polycyclic aromatic hydrocarbons found in tobacco smoke are potent inducers of CYP1A2 so that smokers may require significant modification of medication dosage.[12] A recent warning has been published regarding potentially serious interaction with tizanidine, based on CYP1A2 metabolism.[13] The half-life of caffeine is significantly extended by the use of fluvoxamine, which can cause insomnia and irritability in coffee drinkers.
Fluvoxamine inhibits metabolism of diazepam and phenytoin via CYP2C19 and metabolism of aripiprazole, chlorpromazine, clozapine, haloperidol, olanzapine, perphenazine, risperidone, thioridazine and zuclopenthixol via CYP2D6 as well as of aripiprazole, clozapine, haloperidol, quetiapine and ziprasidone via CYP3A4.[14]
Fluvoxamine has low potential for the drug interactions which are based on inhibition of enzyme Cytochrome P450 CYP2D6, less than most other SSRIs.[15][16][17] Naturally the other SSRIs which are metabolized by CYP2D6 will have more CYP2D6-based interactions with TCAs, antiarrhythmics, B-blockers, phenytoin, opioids and neuroleptics.
The plasma protein binding of fluvoxamine is about 77%. Drugs with low protein binding are less likely to displace other protein bound drugs, and therefore have a lower potential to cause protein binding-related drug interactions.
Fluvoxamine also inhibits CYP2C9.[10][18]
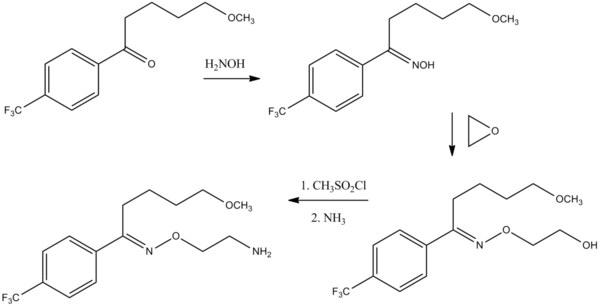
Synthesis
 Luvox (fluvoxamine) 100 mg film-coated scored tablets (AU)
Luvox (fluvoxamine) 100 mg film-coated scored tablets (AU)
Fluvoxamine is one of only two SSRIs (along with alaproclate) to have a monocyclic structure.[19][20]
History
Fluvoxamine was developed by Solvay Pharmaceuticals and was the first non-TCA drug approved by the U.S. Food and Drug Administration (FDA) specifically for the treatment of OCD.[21] It was one of the first SSRI antidepressants to be launched (1984 in Switzerland), and following its FDA approval in 1993, it was launched in the U.S. in December 1994, Australia in February 1999 and Japan in June 1999.[22] At the end of 1995, more than 10 million patients worldwide had been treated with fluvoxamine.[23] Fluvoxamine was the first SSRI to be registered for the treatment of obsessive compulsive disorder in children by the FDA in 1997.[24] Fluvoxamine was the first drug approved for the treatment of social anxiety disorder in Japan in 2005.[25]
In 1999, fluvoxamine came under great public scrutiny after it was discovered that Eric Harris, one of the two teenage shooters involved in the Columbine High School massacre, had been taking the drug. Many immediately pointed fingers at fluvoxamine and its manufacturer Solvay Pharmaceuticals.[26] Sales fell, and Solvay withdrew the medication from the U.S. market in 2002.[27] In 2007, Solvay re-introduced Luvox to the U.S. market, which is now manufactured by Palo Alto, California-based Jazz Pharmaceuticals, Inc., with a generic version of Luvox available from IVAX Pharmaceuticals, Inc. On February 28, 2008, the FDA approved a controlled-release formulation of fluvoxamine for Solvay Pharmaceuticals, to be marketed as Luvox CR.[28][29]
Society and culture
The drug was given visibility in the American television series The Sopranos produced by HBO, in the second season, episode 10. Dr. Jennifer Melfi, the psychiatrist treating the character named Tony Soprano, is prescribed Luvox by her own psychiatrist, to treat a strong inclination toward alcohol.
"Stuttering" John Melendez, formerly of The Howard Stern Show, had taken Luvox for a period of time prior to his departure from the show.
One of two shooters at the Columbine High School massacre, Eric Harris, was taking Luvox. Some analysts, such as psychiatrist Peter Breggin, have argued that this medication may have contributed to Harris's actions. Breggin claimed that side-effects of these drugs include increased aggression, loss of remorse, depersonalization, and mania.
References
- ^ "FDA Advisory Committee Recommends Luvox (Fluvoxamine) Tablets for Obsessive Compulsive Disorder," PRNewswire, 10/18/93
- ^ Stahl, S. Stahl's Essential Psychopharmacology: The Prescriber's Guide. Cambridge University Press. New York, NY. 2009. pp.215
- ^ Karen J. McClellan, David P. Figgitt (Drugs October 2000). "Fluvoxamine An Updated Review of its Use in the Management of Adults with Anxiety Disorders". Adis Drug Evaluation 60 (4): 925–954.
- ^ US-FDA Fluvoxamine Product Insert. March 2005.
- ^ LUVOX® Consumer Medicine Information | Better Health Channel
- ^ Hengeveld VW et al., Waldinger MD; Hengeveld, MW; Zwinderman, AH; Olivier, B (1998). "Effect of SSRI antidepressants on ***: a double blind, randomised, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine and sertraline". Journal of Clinical Psychopharmacology 18 (4): 274–281. doi:10.1097/00004714-199808000-00004. PMID 9690692.
- ^ Hashimoto K et al., Narita N; Hashimoto, K; Tomitaka, S; Minabe, Y (1996). "Interactions of selective reuptake inhibitors with subtypes of sigma receptor in rat brain". Eur J Pharmacol 307 (1): 117–9. doi:10.1016/0014-2999(96)00254-3. PMID 8831113.
- ^ C.Sandner, Carrasco JL; Sandner, C (December 2005). "Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: an overview". International Journal of Clinical Practice 59 (12): 1428–1434. doi:10.1111/j.1368-5031.2005.00681.x. PMID 16351675.
- ^ Barr Laboratories Inc (October 2010). "Fluvoxamine Official FDA information, side effects and uses". Subsection: Fluvoxamine - Clinical Pharmacology -> Pharmacokinetics. http://www.drugs.com/pro/fluvoxamine.html. Retrieved 2011-02-15.
- ^ a b c "Luvox Tablets (Fluvoxamine Maleate) Drug Information: Uses, Side Effects, Drug Interactions and Warnings at RxList". http://www.rxlist.com/luvox-drug.htm. Retrieved 2009-02-17.
- ^ Center for Drug Evaluation and Research, (2000). Fluvoxamine Maleate Tablets. Application Number: 75901, Retrieved July 28, 2008, from http://www.fda.gov/cder/foi/anda/2000/75901_Fluvoxamine%20Maleate_Prntlbl.pdf
- ^ Kroom, Lisa A. (10-01-2007). "Drug Interactions With Smoking". Am J Health-Syst Pharm. (Medscape: American Society of Health-System Pharmacists) 64 (18): 1917–1921. doi:10.2146/ajhp060414. PMID 17823102. http://www.medscape.com/viewarticle/562754_print. Retrieved 2008-01-31.
- ^ Waknine, Yael (April 13, 2007). "Prescribers Warned of Tizanidine Drug Interactions". Medscape News. Medscape. http://www.medscape.com/viewarticle/555194_print. Retrieved 2008-02-01.
- ^ Bondy, Brigitta; Illja Spellmann (2007). "Pharmacogenetics of Antipsychotics: Useful For the Clinician?". Curr Opin Psychiatry (Medscape: Lippincott Williams & Wilkins) 20 (1): 126–130. doi:10.1097/YCO.0b013e328017f69f. PMID 17278909. http://www.medscape.com/viewarticle/552100_print. Retrieved 2008-02-01.
- ^ P., Baumann (1996). "Pharmacokinetic-pharmacodynamic relationship of the Selective serotonin reuptake inhibitors". Clinical Pharmacokinetics 31 (6): 444–469. doi:10.2165/00003088-199631060-00004. PMID 8968657.
- ^ Gill HS, DeVane CL; Gill, HS (1997). "Clinical Pharmacokinetics of Fluvoxamine: applications to dosage regime design". Journal of Clinical Psychiatry 58 (Suppl 5): 7–14. PMID 9184622.
- ^ DeVane, CL (1998). "Translational pharmacokinetics: current issues with newer antidepressants". Depression and Anxiety 8 (Suppl 1): 64–70. doi:10.1002/(SICI)1520-6394(1998)8:1+<64::AID-DA10>3.0.CO;2-S. PMID 9809216.
- ^ "Brain Elimination Half-Life of Fluvoxamine". http://ajp.psychiatryonline.org/cgi/content/full/155/3/380.
- ^ A Wilkinson, M Courtney, A Westlind-Danielsson, G Hallnemo and K E Akerman (December). "Alaproclate acts as a potent, reversible and noncompetitive antagonist of the NMDA receptor coupled ion flow". JPET 271 (3): 1314–1319.
- ^ K L Nicholson and R L Balster (23 April). "Evaluation of the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats". Psychopharmacology.
- ^ Pharmalot | BrainPhysics.com| Medications for OCD.
- ^ Solvay Pharmaceuticals Australia | Luvox.
- ^ Fluvoxamine Product Monograph. 1999.
- ^ "Luvox Approved For Obsessive Compulsive Disorder in Children and Teens". Http://www.pslgroup.com/dg/2261a.htm.
- ^ "Solvay's Fluvoxamine maleate is first drug approved for the treatment of social anxiety disorder in Japan". Http://www.solvaypress.com/pressreleases/0,,33713-2-83,00.htm.
- ^ Pankratz, Howard (January 26, 2007). "Judge: Seal Columbine papers for 25 years". The Denver Post. http://www.denverpost.com/golf/ci_5094436. Retrieved 2008-03-03.
- ^ "Solvay Pharmaceuticals, Inc. Withdraws LUVOX". Http://www.solvaypharmaceuticals-us.com/newsroom/pressreleases/0,,14517-2-0,00.htm.
- ^ "Jazz Pharmaceuticals press release, February 28, 2008 – FDA APPROVES LUVOX CR (FLUVOXAMINE MALEATE) EXTENDED-RELEASE CAPSULES FOR THE TREATMENT OF SOCIAL ANXIETY DISORDER (SAD) AND OBSESSIVE COMPULSIVE DISORDER (OCD)". Archived from the original on 2008-05-26. http://web.archive.org/web/20080526082602/http://www.jazzpharma.com/news.php?id=59. Retrieved 2008-03-14.
- ^ "Luvox CR | Prescribing Info". http://www.luvoxcr.com/LUVOX-CR-PI.pdf. Retrieved 2008-02-21.
Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;• Quercetin;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersExternal links
Categories:- Selective serotonin reuptake inhibitors
- Sigma agonists
- Oximes
- Ethers
- Organofluorides
Wikimedia Foundation. 2010.