- Diisopropyl fluorophosphate
-
Diisopropyl fluorophosphate 

Systematic (IUPAC) name Di(propan-2-yl) phosphorofluoridate Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 55-91-4 
ATC code S01EB07 PubChem CID 5936 DrugBank APRD00763 ChemSpider 5723 
UNII 12UHW9R67N 
KEGG D00043 
ChEBI CHEBI:17941 
ChEMBL CHEMBL1025 
Chemical data Formula C6H14FO3P Mol. mass 184.146 g/mol SMILES eMolecules & PubChem Physical data Melt. point -82 °C (-116 °F) Boiling point 183 °C (361 °F) 1013 mbar  (what is this?) fluorophosphate (verify)
(what is this?) fluorophosphate (verify)Diisopropyl fluorophosphate (DFP, DIFP, diisopropyl phosphorofluoridate) is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine[citation needed] and as an organophosphorus insecticide[citation needed]. It is stable, but undergoes hydrolysis when subjected to moisture, producing hydrofluoric acid. It is known also under names Difluorophate, Diflupyl, Diflurphate, Dyflos, Dyphlos, Fluropryl, Fluostigmine, isofluorophate, isofluorphate, Neoglaucit, PF-3, PF3, T-1703, TL 466, and others.
Contents
Uses in medicine
Diisopropyl fluorophosphate is a parasympathomimetic drug "irreversible anti-cholinesterase" and has been used in ophthalmology as a miotic agent in treatment of chronic glaucoma, as a miotic in veterinary medicine, and as an experimental agent in neuroscience because of its acetylcholinesterase inhibitory properties and ability to induce delayed peripheral neuropathy[citation needed]. It is known as fluostigmine and Dyflos in such uses.
Uses as toxin
The marked toxicity of esters of monofluorophosphoric acid was discovered in 1932, when Willy Lange and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist Bernard Charles Saunders. On his search for compounds to be used as chemical warfare agents, Saunders was inspired by the report by Lange und Krueger and decided to prepare the new homologue which he labeled PF-3. It was much less effective as a chemical weapon than the G series agents. It was often mixed with mustard gas, forming a more effective mixture with significantly lower melting point, resulting in an agent suitable for use in cold weather.
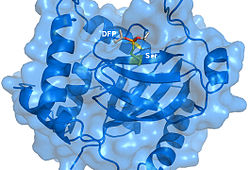 Crystal structure of Herpes Simplex Virus Protease/Inhibitor (DFP) complex. The active site serine (yellow) has undergone phosphonylation resulting in irreversible inhibition. Rendered from PDB 1AT3.
Crystal structure of Herpes Simplex Virus Protease/Inhibitor (DFP) complex. The active site serine (yellow) has undergone phosphonylation resulting in irreversible inhibition. Rendered from PDB 1AT3.
In military research, due to its physical and chemical similarities and comparatively low toxicity, it is used as a simulant of G-agents (GA, GB, GD, GF).
Diisopropyl fluorophosphate is a very potent neurotoxin. Its LD50 in rats is 1.3 mg/kg. It combines with the amino acid serine at the active site of the enzyme acetylcholinesterase, an enzyme that deactivates the neurotransmitter acetylcholine. Neurotransmitters are needed to continue the passage of nerve impulses from one neuron to another across the synapse. Once the impulse has been transmitted, acetylcholinesterase functions to deactivate the acetylcholine almost immediately by breaking it down. If the enzyme is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle contraction. Paralysis occurs and death may result since the respiratory muscles are affected.
DFP also inhibits some proteases. It is a useful additive for protein or cell isolation procedure.
Chemistry
Isoflurophate, the di-iso-propyl ester of fluorophosphoric acid, is made by reacting isopropyl alcohol with phosphorus trichloride, forming di-iso-propylphosphite, which is chlorinated and further reacted with sodium fluoride to replace the chlorine atom with fluorine, thus giving isofluorophate.
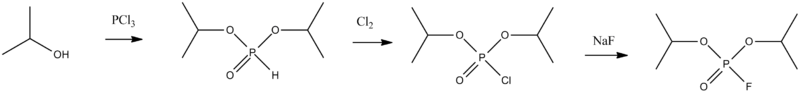
- E.E. Hardy, G.M. Kosoloapoff, U.S. Patent 2,409,039 (1946).
See also
- MAFP - methoxy arachidonoylfluorophosphonate, a mechanistically related inhibitor
References
- Brenner, G. M. (2000): Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6
- Meiers, P. (2006): History of the fluorophosphates
External links
Ophthalmologicals: antiglaucoma preparations and miotics (S01E) Sympathomimetics Parasympathomimetics muscarinicmuscarinic/nicotinicCarbonic anhydrase inhibitors/
(sulfonamides)Beta blocking agents Prostaglandin analogues (F2α) Other agents M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Organophosphate insecticides
- Acetylcholinesterase inhibitors
- Phosphorofluoridates
- Ophthalmology drugs
- Neurotoxins
Wikimedia Foundation. 2010.
Look at other dictionaries:
diisopropyl fluorophosphate — SYN: isofluorphate. * * * diisopropyl fluorophosphate n ISOFLUROPHATE * * * (DFP) di·iso·pro·pyl flu·o·ro·phos·phate (DFP) (di i″so proґpəl fl r″o fosґfāt) a potent irreversible acetylcholinesterase and pseudocholinesterase… … Medical dictionary
diisopropyl fluorophosphate — diizopropilfluorfosfatas statusas T sritis chemija formulė [(CH₃)₂CHO]₂P(=O)F atitikmenys: angl. diisopropyl fluorophosphate rus. диизопропилфторфосфат … Chemijos terminų aiškinamasis žodynas
diisopropyl fluorophosphate — noun Etymology: International Scientific Vocabulary diisopropyl + fluorophosphate : a volatile irritating liquid ester [(CH3)2CH]2PO3F that acts as a nerve gas by inhibiting cholinesterases and as a myotic and that is used chiefly in treating… … Useful english dictionary
Diisopropyl-fluorophosphatase — Identifiers EC number 3.1.8.2 CAS number 9032 18 2 … Wikipedia
DFP — Abbreviation for diisopropyl fluorophosphate. * * * deferiprone; diastolic filling period; diisopropyl fluorophosphate * * * DFP .dē .ef pē n ISOFLUROPHATE * * * diisopropyl fluorophosphate … Medical dictionary
Omethoate — IUPAC name 2 [(Dimethoxyphosphoryl)sulfanyl] N methyl acetamide … Wikipedia
Parathion — IUPAC name O,O Diethyl O (4 nitrophenyl) phosphorothioate … Wikipedia
Malathion — Malathion … Wikipedia
Chlorfenvinphos — IUPAC name [(EZ) 2 Chloro 1 (2,4 dichlorophenyl)ethenyl] diethyl phosphate … Wikipedia
Chlorpyrifos — I … Wikipedia

