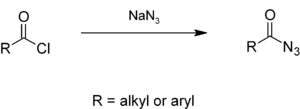- Azide
-
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−. Azide is also a functional group in organic chemistry, RN3.[1]
Sodium azide is found in automobile air bags; it decomposes on heating to give nitrogen gas, which is used to quickly expand the air bag. The antiviral drug zidovudine (AZT) contains an azido group. Some azides are valuable as bioorthogonal chemical reporters.
In organic chemistry, azides are commonly used as a way to introduce an amine group. They are also popular for their participation in the "click reaction", and Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry. That aside, low-molecular weight azides tend to be unstable, hence the need for caution.
Contents
Preparation
The principal source of the azide moiety is sodium azide. Sodium azide is made industrially by the reaction of nitrous oxide, N2O with sodium amide, NaNH2, in liquid ammonia as solvent. The overall stoichiometry is given by [2]
- N2O + 2NaNH2 → NaN3 + NaOH + NH3
Most inorganic and organic azides are prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. As a pseudohalogen compound, sodium azide generally displaces an appropriate leaving group (e.g. Br, I, OTs) to give the azido compound.
Aryl azides may be prepared by displacement of the appropriate diazonium salt with sodium azide, or trimethylsilyl azide; nucleophilic aromatic substitution is also possible, even with chlorides. Anilines and aromatic hydrazines undergo diazotization, as do alkyl amines and hydrazines.[1]
Appropriately functionalized aliphatic compounds undergo nucleophilic substitution with sodium azide. Aliphatic alcohols give azides via a variant of the Mitsunobu reaction, with the use of hydrazoic acid.[1] Hydrazines may also form azides by reaction with sodium nitrite:[3]
- PhNHNH2 → PhN3
Alkyl or aryl acyl chlorides react with sodium azide in aqueous solution to give acyl azides,[4][5] which give isocyanates in the Curtius rearrangement.
The azo-transfer compounds, trifluoromethanesulfonyl azide and imidazole-1-sulfonyl azide, are prepared from sodium azide as well. They react with amines to give the corresponding azides:
- RNH2 → RN3
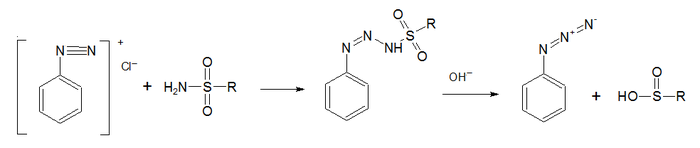
Dutt-Wormall reaction
A classic method for the synthesis of azides is the Dutt-Wormall reaction [6] in which a diazonium salt reacts with a sulfonamide first to a diazoaminosulfinate and then on hydrolysis the azide and a sulfinic acid.[7]
Reactions
Inorganic azides
Most azide salts decompose violently to give nitrogen gas, for example with the sodium and silver azides:
- 2 NaN3 → 2 Na + 3 N2
- 2 AgN3 → 2 Ag + 3 N2
They also liberate toxic hydrazoic acid in the presence of strong acids:
- H+ + N3− → HN3
Azide salts may react with heavy metals or heavy metal compounds to give the corresponding azides, which are more sensitive than sodium azide alone. They decompose with sodium nitrite when acidified. This is a method of destroying residual azides, prior to disposal.[8]
- 2 NaN3 + 2 HNO2 → 3 N2 + 2 NO + 2 NaOH
Many inorganic covalent azides, e.g. chlorine, bromine, and iodine azides, have been described as well.[9]
The azide anion behaves as a nucleophile; it undergoes nucleophilic substitution for both aliphatic and aromatic systems. It reacts with epoxides, causing a ring-opening; it undergoes Michael-like conjugate addition to 1,4-unsaturated carbonyl compounds.[1]
Organic azides
Organic azides engage in useful organic reactions. The terminal nitrogen is mildly nucleophilic. Azides easily extrude diatomic nitrogen, a tendency that is exploited in many reactions such as the Staudinger ligation or the Curtius rearrangement or for example in the synthesis of γ-imino-β-enamino esters.[10][11]
Azides may be reduced to amines by hydrogenolysis[12] or with a phosphine, e.g. triphenylphosphine, in the Staudinger reaction. This reaction allows azides to serve as protected -NH2 synthons:
- RN3 → RNH2
In the azide alkyne Huisgen cycloaddition, organic azides react as 1,3-dipoles, reacting with alkynes to give substituted 1,2,3-triazoles. This reaction is very popular in click chemistry.
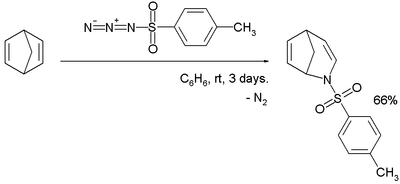
Another azide regular is tosyl azide here in reaction with norbornadiene in a nitrogen insertion reaction:[13]
Azides in coordination chemistry
Azides can be used as sources of the nitrido function in the coordination chemistry of late transition metals, loss of N2 from terminal azide complexes can thus facilitate the formation of unusual oxidation states (see high-valent iron).
Safety
- Azides are explosophores and toxins. Example: tetraazidomethane.
- Sodium azide is toxic (LD50 oral (rat) = 27 mg/kg) and can be absorbed through the skin. It decomposes explosively upon heating to above 275 °C and reacts vigorously with CS2, bromine, nitric acid, dimethyl sulfate, and a series of heavy metals, including copper and lead. In reaction with water or Brønsted acids the highly toxic and explosive hydrogen azide is released.
- Heavy metal azides, such as lead azide are primary high explosives detonable when heated or shaken. Heavy-metal azides are formed when solutions of sodium azide or HN3 vapors come into contact with heavy metals or their salts. Heavy-metal azides can accumulate under certain circumstances, for example, in metal pipelines and on the metal components of diverse equipment (rotary evaporators, freezedrying equipment, cooling traps, water baths, waste pipes), and thus lead to violent explosions.
- Some organic and other covalent azides are classified as highly explosive and toxic (inorganic azides as neurotoxins; azide ions as cytochrome c oxidase inhibitors).
- It has been reported that sodium azide and polymer-bound azide reagents react with dichloromethane and chloroform to form di- and triazidomethane resp., which are both unstable in high concentrations in solution. Various devastating explosions were reported while reaction mixtures were being concentrated on a rotary evaporator. The hazards of diazidomethane (and triazidomethane) have been well documented.[14][15]
- Solid iodoazide is explosive and should not be prepared in the absence of solvent.[16]
See also
References
- ^ a b c d S. Bräse, C. Gil, K. Knepper and V. Zimmermann (2005). "Organic Azides: An Exploding Diversity of a Unique Class of Compounds". Angewandte Chemie International Edition 44 (33): 5188–5240. doi:10.1002/anie.200400657. PMID 16100733.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. p. 433. ISBN 0080379419.
- ^ R. O. Lindsay and C. F. H. Allen (1955), "Phenyl azide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0710; Coll. Vol. 3: 710
- ^ C. F. H. Allen and Alan Bell, "Undecyl isocyanate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0846; Coll. Vol. 3: 846
- ^ Jon Munch-Petersen (1963), "m-Nitrobenzazide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0715; Coll. Vol. 4: 715
- ^ Pavitra Kumar Dutt, Hugh Robinson Whitehead and Arthur Wormall (1921). "CCXLI.—The action of diazo-salts on aromatic sulphonamides. Part I". J. Chem. Soc., Trans. 119: 2088. doi:10.1039/CT9211902088.
- ^ Name Reactions: A Collection of Detailed Reaction Mechanisms by Jie Jack Li Published 2003 Springer ISBN 3540402039
- ^ Committee on Prudent Practices for Handling, Storage, and Disposal of Chemicals in Laboratories, Board on Chemical Sciences and Technology, Commission on Physical Sciences, Mathematics, and Applications, National Research Council. (1995). Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. ISBN 0309052297. http://books.nap.edu/openbook.php?record_id=4911&page=165.
- ^ I. C. Tornieporth-Oetting and T. M. Klapötke (1995). "Covalent Inorganic Azides". Angewandte Chemie International Edition in English 34 (5): 511–520. doi:10.1002/anie.199505111.
- ^ Mangelinckx, S.; Van Vooren, P.; De Clerck, D.; Fülöp, F.; De Kimpea, N. (2006). "An efficient synthesis of γ-imino- and γ-amino-β-enamino esters". Arkivoc (iii): 202–209. http://www.arkat-usa.org/get-file/19626/.
- ^ Reaction conditions: a) sodium azide 4 eq., acetone, 18 hours reflux 92% chemical yield b) isopropyl amine, titanium tetrachloride, diethyl ether 14 hr reflux 83% yield. Azide 2 is formed in a nucleophilic aliphatic substitution reaction displacing chlorine in 1 by the azide anion. The ketone reacts with the amine to an imine which tautomerizes to the enamine in 4. In the next rearrangement reaction nitrogen is expulsed and a proton transferred to 6. The last step is another tautomerization with the formation of the enamine 7 as a mixture of cis and trans isomers
- ^ http://www.organic-chemistry.org/synthesis/N1H/reductionsazides.shtm
- ^ Damon D. Reed and Stephen C. Bergmeier (2007). "A Facile Synthesis of a Polyhydroxylated 2-Azabicyclo[3.2.1]octane". J. Org. Chem. 72 (3): 1024–6. doi:10.1021/jo0619231. PMID 17253828.
- ^ M. S. Alfred Hassner (1986). "Synthesis of Alkyl Azides with a Polymeric Reagent". Angewandte Chemie International Edition in English 25 (5): 478–479. doi:10.1002/anie.198604781.
- ^ A. Hassner, M. Stern, H. E. Gottlieb and F. Frolow (1990). "Synthetic methods. 33. Utility of a polymeric azide reagent in the formation of di- and triazidomethane. Their NMR spectra and the x-ray structure of derived triazoles". J. Org. Chem. 55 (8): 2304–2306. doi:10.1021/jo00295a014.
- ^ L. Marinescu, J. Thinggaard, I. B. Thomsen and M. Bols (2003). "Radical Azidonation of Aldehydes". J. Org. Chem. 68 (24): 9453–9455. doi:10.1021/jo035163v. PMID 14629171.
External links
Categories:- Functional groups
- Azides
Wikimedia Foundation. 2010.