- Sulfur dichloride
-
Sulfur dichloride 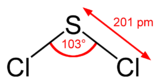

 Sulpur dichloride
Sulpur dichloride
Sulfur(II) chloride
DichlorosulfaneOther namesSulpur chlorideIdentifiers CAS number 10545-99-0 
EC number 234-129-0 UN number 1828 RTECS number WS4500000 Jmol-3D images Image 1 - ClSCl
Properties Molecular formula SCl2 Molar mass 102.97 g mol−1 Appearance red liquid with pungent odour Density 1.621 g cm−3, liquid Melting point −121.0 °C, 152.2 K, -185.8 °F
Boiling point 59 °C, 332 K, 138 °F (decomp.)
Solubility in water hydrolysis Refractive index (nD) 1.5570 Structure Coordination
geometryC2v Molecular shape Bent Hazards MSDS ICSC 1661 EU Index 016-013-00-X EU classification Corrosive (C)
Irritant (Xi)
Dangerous for the environment (N)R-phrases R14, R34, R37, R50 S-phrases (S1/2), S26, S45, S61 NFPA 704 Autoignition
temperature234 °C Related compounds Related Disulfur dichloride
Thionyl chloride
Sulfuryl chlorideRelated compounds Sulfur tetrafluoride
Sulfur hexafluoride
Disulfur dibromide dichloride (verify) (what is:
dichloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common. It is used as a precursor to organosulfur compounds.[1]
Contents
Chlorination of sulfur
SCl2 is produced by the chlorination of either elemental sulfur or disulfur dichloride. The process occurs in a series of steps, some of which are:
-
- S8 + 4 Cl2 → 4 S2Cl2; ΔH = −58.2 kJ/mol
- S2Cl2 + Cl2 → 2 SCl2; ΔH = −40.6 kJ/mol
The addition of Cl2 to S2Cl2 has been proposed to proceed via a mixed valence intermediate Cl3S-SCl. SCl2 undergoes even further chlorination to give SCl4, but this species is unstable at near room temperature. It is likely that several SxCl2 exist where x > 2.
Disulfur dichloride, S2Cl2, is the most common impurity in SCl2. Separation of SCl2 from S2Cl2 is possible via distillation with PCl3 to form an azeotrope of 99% purity
Use of SCl2 in chemical synthesis
- SCl2 is used in organic synthesis. It adds to alkenes to give chloride-substituted thioethers. Illustrative applications are its addition to 1,5-cyclooctadiene to give a bicyclic thioether[2] and ethylene to give sulfur mustard S(CH2CH2Cl)2.[3]
SCl2 is also a precursor to several inorganic sulfur compounds. Treatment with fluoride salts gives SF4. Reaction with ammonia affords sulfur nitrides related to S4N4. With H2S, SCl2 reacts to give "lower" sulfanes such as S3H2.
Safety considerations
SCl2 hydrolyzes with release of HCl. Old samples contain Cl2.
References
- ^ Schmidt, M.; Siebert, W. "Sulphur" Comprehensive Inorganic Chemistry Vol. 2, ed. A.F. Trotman-Dickenson. 1973.
- ^ Bishop, Roger (1992), "9-Thiabicyclo[3.3.1]nonane-2,6-dione", Org. Synth. 70: 120, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv9p0692; Coll. Vol. 9: 692
- ^ R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0 471 95512 4.
Categories:- Sulfur chlorides
Wikimedia Foundation. 2010.

