- Stem cell
-
Stem cell 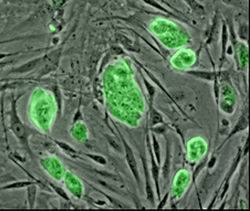
Mouse embryonic stem cells with fluorescent marker 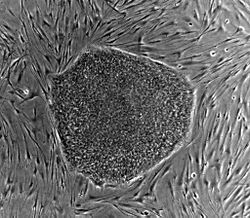
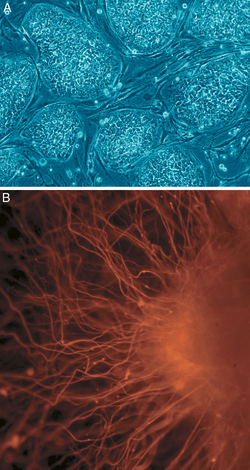
Human embryonic stem cell colony on mouse embryonic fibroblast feeder layer Latin cellula precursoria Code TH H2.00.01.0.00001 This article is about the cell type. For the medical therapy, see Stem Cell Treatments
Stem cells are biological cells found in all multicellular organisms, that can divide (through mitosis) and differentiate into diverse specialized cell types and can self-renew to produce more stem cells. In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cells, which are found in various tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing adult tissues. In a developing embryo, stem cells can differentiate into all the specialized cells (these are called pluripotent cells), but also maintain the normal turnover of regenerative organs, such as blood, skin, or intestinal tissues.
Stem cells can now be artificially grown and transformed into specialized cell types with characteristics consistent with cells of various tissues such as muscles or nerves through cell culture. Highly plastic adult stem cells are routinely used in medical therapies. Stem cells can be taken from a variety of sources, including umbilical cord blood and bone marrow. Embryonic cell lines and autologous embryonic stem cells generated through therapeutic cloning have also been proposed as promising candidates for future therapies.[1] Research into stem cells grew out of findings by Ernest A. McCulloch and James E. Till at the University of Toronto in the 1960s.[2][3]
There are three sources of autologous adult stem cells: 1) Bone marrow, which requires extraction by harvesting, that is, drilling into bone (typically the femur or iliac crest), 2) Adipose tissue (lipid cells), which requires extraction by liposuction, and 3) Blood, which requires extraction through pheresis, wherein blood is drawn from the donor, (similar to a blood donation) passed through a machine that extracts the stem cells and returns other portions of the blood to the donor.
Of all stem cell types, autologous harvesting involves the least risk. By definition, autologous cells are obtained from one's own body, just as one may bank his or her own blood for elective surgical procedures.
Contents
Properties
The classical definition of a stem cell requires that it possess two properties:
- Self-renewal: the ability to go through numerous cycles of cell division while maintaining the undifferentiated state.
- Potency: the capacity to differentiate into specialized cell types. In the strictest sense, this requires stem cells to be either totipotent or pluripotent—to be able to give rise to any mature cell type, although multipotent or unipotent progenitor cells are sometimes referred to as stem cells.Apart from this it is being said that stem cells function is regulated in a feed back mechanism.
Self-renewal
Two mechanisms to ensure that the stem cell population is maintained exist:
- Obligatory asymmetric replication: a stem cell divides into one father cell that is identical to the original stem cell, and another daughter cell that is differentiated
- Stochastic differentiation: when one stem cell develops into two differentiated daughter cells, another stem cell undergoes mitosis and produces two stem cells identical to the original.
Potency definitions
Main article: Cell potencyPotency specifies the differentiation potential (the potential to differentiate into different cell types) of the stem cell.[4]
- Totipotent (a.k.a omnipotent) stem cells can differentiate into embryonic and extraembryonic cell types. Such cells can construct a complete, viable organism.[4] These cells are produced from the fusion of an egg and sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent.[5]
- Pluripotent stem cells are the descendants of totipotent cells and can differentiate into nearly all cells,[4] i.e. cells derived from any of the three germ layers.[6]
- Multipotent stem cells can differentiate into a number of cells, but only those of a closely related family of cells.[4]
- Oligopotent stem cells can differentiate into only a few cells, such as lymphoid or myeloid stem cells.[4]
- Unipotent cells can produce only one cell type, their own,[4] but have the property of self-renewal, which distinguishes them from non-stem cells (e.g., muscle stem cells).
Identification
The practical definition of a stem cell is the functional definition—a cell that has the potential to regenerate tissue over a lifetime. For example, the defining test for a bone marrow or hematopoietic stem cell (HSC) is the ability to transplant one cell and save an individual without HSCs. In this case, a stem cell must be able to produce new blood cells and immune cells over a long term, demonstrating potency. It should also be possible to isolate stem cells from the transplanted individual, which can themselves be transplanted into another individual without HSCs, demonstrating that the stem cell was able to self-renew.
Properties of stem cells can be illustrated in vitro, using methods such as clonogenic assays, in which single cells are assessed for their ability to differentiate and self-renew.[7][8] Stem cells can also be isolated by their possession of a distinctive set of cell surface markers. However, in vitro culture conditions can alter the behavior of cells, making it unclear whether the cells will behave in a similar manner in vivo. There is considerable debate as to whether some proposed adult cell populations are truly stem cells.
Embryonic
Main article: Embryonic stem cellEmbryonic stem cell lines (ES cell lines) are cultures of cells derived from the epiblast tissue of the inner cell mass (ICM) of a blastocyst or earlier morula stage embryos.[9] A blastocyst is an early stage embryo—approximately four to five days old in humans and consisting of 50–150 cells. ES cells are pluripotent and give rise during development to all derivatives of the three primary germ layers: ectoderm, endoderm and mesoderm. In other words, they can develop into each of the more than 200 cell types of the adult body when given sufficient and necessary stimulation for a specific cell type. They do not contribute to the extra-embryonic membranes or the placenta. The endoderm is composed of the entire gut tube and the lungs, the ectoderm gives rise to the nervous system and skin, and the mesoderm gives rise to muscle, bone, blood—in essence, everything else that connects the endoderm to the ectoderm.
Nearly all research to date has made use of mouse embryonic stem cells (mES) or human embryonic stem cells (hES). Both have the essential stem cell characteristics, yet they require very different environments in order to maintain an undifferentiated state. Mouse ES cells are grown on a layer of gelatin and require the presence of leukemia inhibitory factor (LIF).[10] Human ES cells are grown on a feeder layer of mouse embryonic fibroblasts (MEFs) and require the presence of basic fibroblast growth factor (bFGF or FGF-2).[11] Without optimal culture conditions or genetic manipulation,[12] embryonic stem cells will rapidly differentiate.
A human embryonic stem cell is also defined by the presence of several transcription factors and cell surface proteins. The transcription factors Oct-4, Nanog, and Sox2 form the core regulatory network that ensures the suppression of genes that lead to differentiation and the maintenance of pluripotency.[13] The cell surface antigens most commonly used to identify hES cells are the glycolipids stage specific embryonic antigen 3 and 4 and the keratan sulfate antigens Tra-1-60 and Tra-1-81. The molecular definition of a stem cell includes many more proteins and continues to be a topic of research.[14]
There are currently no approved treatments using embryonic stem cells. The first human trial was approved by the US Food and Drug Administration in January 2009.[15] However, the human trial had not yet been initiated until October 13, 2010 in Atlanta for spinal injury victims. ES cells, being pluripotent cells, require specific signals for correct differentiation—if injected directly into another body, ES cells will differentiate into many different types of cells, causing a teratoma. Differentiating ES cells into usable cells while avoiding transplant rejection are just a few of the hurdles that embryonic stem cell researchers still face.[16] Many nations currently have moratoria on either ES cell research or the production of new ES cell lines. Because of their combined abilities of unlimited expansion and pluripotency, embryonic stem cells remain a theoretically potential source for regenerative medicine and tissue replacement after injury or disease.
Fetal
The primitive stem cells located in the organs of fetuses are referred to as fetal stem cells.[17]
Adult
Main article: Adult stem cellAlso known as somatic (from Greek Σωματικóς, "of the body") stem cells and germline (giving rise to gametes) stem cells, they can be found in children, as well as adults.[18]
Pluripotent adult stem cells are rare and generally small in number but can be found in a number of tissues including umbilical cord blood.[19] A great deal of adult stem cell research to date has had the aim of characterizing the capacity of the cells to divide or self-renew indefinitely and their differentiation potential.[20] In mice, pluripotent stem cells are directly generated from adult fibroblast cultures. Unfortunately, many mice do not live long with stem cell organs.[21]
Most adult stem cells are lineage-restricted (multipotent) and are generally referred to by their tissue origin (mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell, dental pulp stem cell, etc.).[22][23]
Adult stem cell treatments have been successfully used for many years to treat leukemia and related bone/blood cancers through bone marrow transplants.[24] Adult stem cells are also used in veterinary medicine to treat tendon and ligament injuries in horses.[25]
The use of adult stem cells in research and therapy is not as controversial as the use of embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo. Additionally, in instances where adult stem cells are obtained from the intended recipient (an autograft), the risk of rejection is essentially non-existent. Consequently, more US government funding is being provided for adult stem cell research.[26]
An extremely rich source for adult mesenchymal stem cells is the developing tooth bud of the mandibular third molar.[27] The stem cells eventually form enamel (ectoderm), dentin, periodontal ligament, blood vessels, dental pulp, nervous tissues, and a minimum of 29 different end organs. Because of extreme ease in collection at 8–10 years of age before calcification and minimal to no morbidity, these will probably constitute a major source of cells for personal banking, research and current or future therapies. These stem cells have been shown capable of producing hepatocytes.[citation needed]
Amniotic
Multipotent stem cells are also found in amniotic fluid. These stem cells are very active, expand extensively without feeders and are not tumorigenic. Amniotic stem cells are multipotent and can differentiate in cells of adipogenic, osteogenic, myogenic, endothelial, hepatic and also neuronal lines.[28] All over the world, universities and research institutes are studying amniotic fluid to discover all the qualities of amniotic stem cells, and scientists such as Anthony Atala[29][30] and Giuseppe Simoni [31][32][33] have discovered important results.
Use of stem cells from amniotic fluid overcomes the ethical objections to using human embryos as a source of cells. Roman Catholic teaching forbids the use of embryonic stem cells in experimentation; accordingly, the Vatican newspaper "Osservatore Romano" called amniotic stem cell "the future of medicine".[34]
It is possible to collect amniotic stem cells for donors or for autologuous use: the first US amniotic stem cells bank [35][36] opened in 2009 in Medford, MA, by Biocell Center Corporation [37][38][39] and collaborates with various hospitals and universities all over the world.[40]
Induced pluripotent
Main article: Induced pluripotent stem cellThese are not adult stem cells, but rather reprogrammed cells (e.g. epithelial cells) given pluripotent capabilities. Using genetic reprogramming with protein transcription factors, pluripotent stem cells equivalent to embryonic stem cells have been derived from human adult skin tissue.[41][42][43] Shinya Yamanaka and his colleagues at Kyoto University used the transcription factors Oct3/4, Sox2, c-Myc, and Klf4[41] in their experiments on cells from human faces. Junying Yu, James Thomson, and their colleagues at the University of Wisconsin–Madison used a different set of factors, Oct4, Sox2, Nanog and Lin28,[41] and carried out their experiments using cells from human foreskin.
As a result of the success of these experiments, Ian Wilmut, who helped create the first cloned animal Dolly the Sheep, has announced that he will abandon nuclear transfer as an avenue of research.[44]
Frozen blood samples can be used as a source of induced pluripotent stem cells, opening a new avenue for obtaining the valued cells.[45]
Lineage
Main article: Stem cell lineTo ensure self-renewal, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives rise to two identical daughter cells both endowed with stem cell properties. Asymmetric division, on the other hand, produces only one stem cell and a progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before terminally differentiating into a mature cell. It is possible that the molecular distinction between symmetric and asymmetric divisions lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells.[46]
An alternative theory is that stem cells remain undifferentiated due to environmental cues in their particular niche. Stem cells differentiate when they leave that niche or no longer receive those signals. Studies in Drosophila germarium have identified the signals dpp and adherens junctions that prevent germarium stem cells from differentiating.[47][48]
Main article: Induced Pluripotent Stem CellThe signals that lead to reprogramming of cells to an embryonic-like state are also being investigated. These signal pathways include several transcription factors including the oncogene c-Myc. Initial studies indicate that transformation of mice cells with a combination of these anti-differentiation signals can reverse differentiation and may allow adult cells to become pluripotent.[21] However, the need to transform these cells with an oncogene may prevent the use of this approach in therapy.
Challenging the terminal nature of cellular differentiation and the integrity of lineage commitment, it was recently determined that the somatic expression of combined transcription factors can directly induce other defined somatic cell fates; researchers identified three neural-lineage-specific transcription factors that could directly convert mouse fibroblasts (skin cells) into fully functional neurons. This "induced neurons" (iN) cell research inspires the researchers to induce other cell types implies that all cells are totipotent: with the proper tools, all cells may form all kinds of tissue.[49]
Treatments
Main article: Stem cell treatments Diseases and conditions where stem cell treatment is promising or emerging.[50] Bone marrow transplantation is, as of 2009, the only established use of stem cells.
Diseases and conditions where stem cell treatment is promising or emerging.[50] Bone marrow transplantation is, as of 2009, the only established use of stem cells.
Medical researchers believe that stem cell therapy has the potential to dramatically change the treatment of human disease. A number of adult stem cell therapies already exist, particularly bone marrow transplants that are used to treat leukemia.[51] In the future, medical researchers anticipate being able to use technologies derived from stem cell research to treat a wider variety of diseases including cancer, Parkinson's disease, spinal cord injuries, Amyotrophic lateral sclerosis, multiple sclerosis, and muscle damage, amongst a number of other impairments and conditions.[52][53] However, there still exists a great deal of social and scientific uncertainty surrounding stem cell research, which could possibly be overcome through public debate and future research, and further education of the public.
One concern of treatment is the risk that transplanted stem cells could form tumors and become cancerous if cell division continues uncontrollably.[54]
Stem cells are widely studied, for their potential therapeutic use and for their inherent interest.[55]
Supporters of embryonic stem cell research argue that such research should be pursued because the resultant treatments could have significant medical potential. It has been proposed that surplus embryos created for in vitro fertilization could be donated with consent and used for the research.
The recent development of iPS cells has been called a bypass of the legal controversy. Laws limiting the destruction of human embryos have been credited for being the reason for development of iPS cells, but it is still not completely clear whether hiPS cells are equivalent to hES cells. Recent work demonstrates hotspots of aberrant epigenomic reprogramming in hiPS cells (Lister, R., et al., 2011).
Research patents
The patents covering a lot of work on human embryonic stem cells are owned by the Wisconsin Alumni Research Foundation (WARF). WARF does not charge academics to study human stem cells but does charge commercial users. WARF sold Geron Corp. exclusive rights to work on human stem cells but later sued Geron Corp. to recover some of the previously sold rights. The two sides agreed that Geron Corp. would keep the rights to only three cell types. In 2001, WARF came under public pressure to widen access to human stem-cell technology.[56]
A request for reviewing the WARF patents 5,843,780; 6,200,806; 7,029,913 US Patent and Trademark Office were filed by non-profit patent-watchdogs The Foundation for Taxpayer & Consumer Rights, and the Public Patent Foundation as well as molecular biologist Jeanne Loring of the Burnham Institute. According to them, two of the patents granted to WARF are invalid because they cover a technique published in 1993 for which a patent had already been granted to an Australian researcher. Another part of the challenge states that these techniques, developed by James A. Thomson, are rendered obvious by a 1990 paper and two textbooks. Based on this challenge, patent 7,029,913 has been rejected in 2010. The two remaining hES WARF patents are due to expire in 2015.
The outcome of this legal challenge is particularly relevant to the Geron Corp. as it can only license patents that are upheld.[57]
Key research events
- 1908: The term "stem cell" was proposed for scientific use by the Russian histologist Alexander Maksimov (1874–1928) at congress of hematologic society in Berlin. It postulated existence of haematopoietic stem cells.
- 1960s: Joseph Altman and Gopal Das present scientific evidence of adult neurogenesis, ongoing stem cell activity in the brain; like André Gernez, their reports contradict Cajal's "no new neurons" dogma and are largely ignored.
- 1963: McCulloch and Till illustrate the presence of self-renewing cells in mouse bone marrow.
- 1968: Bone marrow transplant between two siblings successfully treats SCID.
- 1978: Haematopoietic stem cells are discovered in human cord blood.
- 1981: Mouse embryonic stem cells are derived from the inner cell mass by scientists Martin Evans, Matthew Kaufman, and Gail R. Martin. Gail Martin is attributed for coining the term "Embryonic Stem Cell".
- 1992: Neural stem cells are cultured in vitro as neurospheres.
- 1997: Leukemia is shown to originate from a haematopoietic stem cell, the first direct evidence for cancer stem cells.
- 1998: James Thomson and coworkers derive the first human embryonic stem cell line at the University of Wisconsin–Madison.[58]
- 1998: John Gearhart (Johns Hopkins University) extracted germ cells from fetal gonadal tissue (primordial germ cells) before developing pluripotent stem cell lines from the original extract.
- 2000s: Several reports of adult stem cell plasticity are published.
- 2001: Scientists at Advanced Cell Technology clone first early (four- to six-cell stage) human embryos for the purpose of generating embryonic stem cells.[59]
- 2003: Dr. Songtao Shi of NIH discovers new source of adult stem cells in children's primary teeth.[60]
- 2004–2005: Korean researcher Hwang Woo-Suk claims to have created several human embryonic stem cell lines from unfertilised human oocytes. The lines were later shown to be fabricated.
- 2005: Researchers at Kingston University in England claim to have discovered a third category of stem cell, dubbed cord-blood-derived embryonic-like stem cells (CBEs), derived from umbilical cord blood. The group claims these cells are able to differentiate into more types of tissue than adult stem cells.
- 2005: Researchers at UC Irvine's Reeve-Irvine Research Center are able to partially restore the ability of rats with paralyzed spines to walk through the injection of human neural stem cells.[61]
- August 2006: Mouse Induced pluripotent stem cells: the journal Cell publishes Kazutoshi Takahashi and Shinya Yamanaka.[62]
- October 2006: Scientists at Newcastle University in England create the first ever artificial liver cells using umbilical cord blood stem cells.[63][64]
- January 2007: Scientists at Wake Forest University led by Dr. Anthony Atala and Harvard University report discovery of a new type of stem cell in amniotic fluid.[65] This may potentially provide an alternative to embryonic stem cells for use in research and therapy.[66]
- June 2007: Research reported by three different groups shows that normal skin cells can be reprogrammed to an embryonic state in mice.[67] In the same month, scientist Shoukhrat Mitalipov reports the first successful creation of a primate stem cell line through somatic cell nuclear transfer[68]
- October 2007: Mario Capecchi, Martin Evans, and Oliver Smithies win the 2007 Nobel Prize for Physiology or Medicine for their work on embryonic stem cells from mice using gene targeting strategies producing genetically engineered mice (known as knockout mice) for gene research.[69]
- November 2007: Human induced pluripotent stem cells: Two similar papers released by their respective journals prior to formal publication: in Cell by Kazutoshi Takahashi and Shinya Yamanaka, "Induction of pluripotent stem cells from adult human fibroblasts by defined factors",[70] and in Science by Junying Yu, et al., from the research group of James Thomson, "Induced pluripotent stem cell lines derived from human somatic cells":[71] pluripotent stem cells generated from mature human fibroblasts. It is possible now to produce a stem cell from almost any other human cell instead of using embryos as needed previously, albeit the risk of tumorigenesis due to c-myc and retroviral gene transfer remains to be determined.
- January 2008: Robert Lanza and colleagues at Advanced Cell Technology and UCSF create the first human embryonic stem cells without destruction of the embryo[72]
- January 2008: Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts[73]
- February 2008: Generation of pluripotent stem cells from adult mouse liver and stomach: these iPS cells seem to be more similar to embryonic stem cells than the previously developed iPS cells and not tumorigenic, moreover genes that are required for iPS cells do not need to be inserted into specific sites, which encourages the development of non-viral reprogramming techniques.[74]
- March 2008-The first published study of successful cartilage regeneration in the human knee using autologous adult mesenchymal stem cells is published by clinicians from Regenerative Sciences[75]
- October 2008: Sabine Conrad and colleagues at Tübingen, Germany generate pluripotent stem cells from spermatogonial cells of adult human testis by culturing the cells in vitro under leukemia inhibitory factor (LIF) supplementation.[76]
- 30 October 2008: Embryonic-like stem cells from a single human hair.[77]
- 1 March 2009: Andras Nagy, Keisuke Kaji, et al. discover a way to produce embryonic-like stem cells from normal adult cells by using a novel "wrapping" procedure to deliver specific genes to adult cells to reprogram them into stem cells without the risks of using a virus to make the change.[78][79][80] The use of electroporation is said to allow for the temporary insertion of genes into the cell.[81][82][83][84]
- 28 May 2009 Kim et al. announced that they had devised a way to manipulate skin cells to create patient specific "induced pluripotent stem cells" (iPS), claiming it to be the 'ultimate stem cell solution'.[85]
- 11 October 2010 First trial of embryonic stem cells in humans.[86]
- 25 October 2010: Ishikawa et al. write in the Journal of Experimental Medicine that research shows that transplanted cells that contain their new host's nuclear DNA could still be rejected by the invidual's immune system due to foreign mitochondrial DNA. Tissues made from a person's stem cells could therefore be rejected, because mitochondrial genomes tend to accumulate mutations.[87]
- 2011: Israeli scientist Inbar Friedrich Ben-Nun led a team which produced the first stem cells from endangered species, a breakthrough that could save animals in danger of extinction.[88]
See also
- Neural stem cell
- Cancer stem cell
- Stem cell controversy
- Dental pulp stem cells
- Human genome
- Induced Pluripotent Stem Cell (iPS Cell)
- Meristem
- Partial cloning
- Plant stem cells
- Stem cell marker
- Stem Cell Network
- Boyd Melson, boxer who donates all his earnings to stem cell research
- Cell bank
References
- ^ Tuch BE (2006). "Stem cells—a clinical update". Australian Family Physician 35 (9): 719–21. PMID 16969445.
- ^ Becker AJ, McCulloch EA, Till JE (1963). "Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells". Nature 197 (4866): 452–4. doi:10.1038/197452a0. PMID 13970094.
- ^ Siminovitch L, McCulloch EA, Till JE (1963). "The distribution of colony-forming cells among spleen colonies". Journal of Cellular and Comparative Physiology 62 (3): 327–36. doi:10.1002/jcp.1030620313. PMID 14086156.
- ^ a b c d e f Hans R. Schöler (2007). "The Potential of Stem Cells: An Inventory". In Nikolaus Knoepffler, Dagmar Schipanski, and Stefan Lorenz Sorgner. Humanbiotechnology as Social Challenge. Ashgate Publishing, Ltd. p. 28. ISBN 0754657558.
- ^ Mitalipov S, Wolf D (2009). "Totipotency, pluripotency and nuclear reprogramming". Adv. Biochem. Eng. Biotechnol. 114: 185–99. doi:10.1007/10_2008_45. PMC 2752493. PMID 19343304. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2752493.
- ^ Ulloa-Montoya F, Verfaillie CM, Hu WS (Jul 2005). "Culture systems for pluripotent stem cells". J Biosci Bioeng. 100 (1): 12–27. doi:10.1263/jbb.100.12. PMID 16233846.
- ^ Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA (1974). "Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method". Experimental Hematology 2 (2): 83–92. ISSN 0301-472X. PMID 4455512.
- ^ Friedenstein AJ, Gorskaja JF, Kulagina NN (1976). "Fibroblast precursors in normal and irradiated mouse hematopoietic organs". Experimental Hematology 4 (5): 267–74. PMID 976387.
- ^ "New Stem-Cell Procedure Doesn't Harm Embryos, Company Claims". Fox News. 2006-08-24. http://www.foxnews.com/story/0,2933,210078,00.html. Retrieved 2010-02-28.
- ^ "Mouse Embryonic Stem (ES) Cell Culture-Current Protocols in Molecular Biology". https://catalog.invitrogen.com/index.cfm?fuseaction=iProtocol.unitSectionTree&treeNodeID=9E662600C6C10276D8E040E99EA33BB0.[dead link]
- ^ "Culture of Human Embryonic Stem Cells (hESC)". National Institutes of Health. http://stemcells.nih.gov/research/NIHresearch/scunit/culture.asp. Retrieved 2010-03-07.
- ^ Chambers I, Colby D, Robertson M, et al. (2003). "Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells". Cell 113 (5): 643–55. doi:10.1016/S0092-8674(03)00392-1. PMID 12787505.
- ^ Boyer LA, Lee TI, Cole MF, et al. (2005). "Core transcriptional regulatory circuitry in human embryonic stem cells". Cell 122 (6): 947–56. doi:10.1016/j.cell.2005.08.020. PMC 3006442. PMID 16153702. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3006442.
- ^ Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. (2007). "Characterization of human embryonic stem cell lines by the International Stem Cell Initiative". Nat. Biotechnol 25 (7): 803–16. doi:10.1038/nbt1318. PMID 17572666.
- ^ Ron Winslow (2009). "First Embryonic Stem-Cell Trial Gets Approval from the FDA". The Wall Street Journal. 23 January 2009.
- ^ Wu DC, Boyd AS, Wood KJ (2007). "Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine". Front Biosci 12 (8–12): 4525–35. doi:10.2741/2407. PMID 17485394.
- ^ Ariff Bongso; Eng Hin Lee (2005). "Stem cells: their definition, classification and sources". In Ariff Bongso; Eng Hin Lee. Stem Cells: From Benchtop to Bedside. World Scientific. pp. 5. ISBN 981-256-126-9. OCLC 443407924.
- ^ Jiang Y, Jahagirdar BN, Reinhardt RL, et al. (2002). "Pluripotency of mesenchymal stem cells derived from adult marrow". Nature 418 (6893): 41–9. doi:10.1038/nature00870. PMID 12077603.
- ^ Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M (2007). "A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues". Leukemia 21 (5): 860–7. doi:10.1038/sj.leu.2404630. PMID 17344915.
- ^ Gardner RL (2002). "Stem cells: potency, plasticity and public perception". Journal of Anatomy 200 (3): 277–82. doi:10.1046/j.1469-7580.2002.00029.x. PMC 1570679. PMID 12033732. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1570679.
- ^ a b Takahashi K, Yamanaka S (2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell 126 (4): 663–76. doi:10.1016/j.cell.2006.07.024. PMID 16904174.
- ^ Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC (2006). "Review: ex vivo engineering of living tissues with adult stem cells". Tissue Eng 12 (11): 3007–19. doi:10.1089/ten.2006.12.3007. PMID 17518617.
- ^ Gimble JM, Katz AJ, Bunnell BA (2007). "Adipose-derived stem cells for regenerative medicine". Circ Res 100 (9): 1249–60. doi:10.1161/01.RES.0000265074.83288.09. PMID 17495232.
- ^ "Bone Marrow Transplant". http://www.ucsfchildrenshospital.org/treatments/leukemia_treatment_options/index.html.
- ^ Kane, Ed (2008-05-01). "Stem-cell therapy shows promise for horse soft-tissue injury, disease". DVM Newsmagazine. http://veterinarynews.dvm360.com/dvm/Equine+Medicine/Stem-cell-therapy-shows-promise-for-horse-soft-tis/ArticleStandard/Article/detail/515503. Retrieved 2008-06-12.
- ^ "Stem Cell FAQ". US Department of Health and Human Services. 2004. http://www.hhs.gov/news/press/2004pres/20040714b.html. Retrieved 2010-03-07.[dead link]
- ^ Huang GT, Gronthos S, Shi S (2009 Sep). "Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine". J Dent Res 88 (9): 792–806. doi:10.1177/0022034509340867. PMC 2830488. PMID 19767575. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2830488.
- ^ P. De Coppi, G Barstch, Anthony Atala (2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biothecnology 25 (5): 100–106. doi:10.1038/nbt1274. PMID 17206138.
- ^ NewsHour with Jim Lehrer. "Online NewsHour: Update | Amniotic Fluid Yields Stem Cells | January 8, 2007". PBS. http://www.pbs.org/newshour/bb/health/jan-june07/cell_01-08.html. Retrieved 2010-03-14.
- ^ "Public : Stem Cell Briefings". ISSCR. 2008-03-21. http://www.isscr.org/public/briefings/amniotic.htm. Retrieved 2010-03-14.
- ^ National Institute of Healt
- ^ (Lancet, 1983)
- ^ "Biocell picks Massachusetts to house North American headquarters – Related Stories – BIO SmartBrief". Smartbrief.com. http://www.smartbrief.com/news/bio/storyDetails.jsp?issueid=10998D4A-47C0-451B-B35A-754969D7EC5A©id=876B286F-19F6-4013-95AC-9744F69B7AD2&brief=bio&sb_code=rss&&campaign=rss. Retrieved 2010-03-14.
- ^ "Vatican newspaper calls new stem cell source 'future of medicine' :: Catholic News Agency (CNA)". Catholic News Agency. 2010-02-03. http://www.catholicnewsagency.com/news/vatican_newspaper_calls_new_stem_cell_source_future_of_medicine/. Retrieved 2010-03-14.
- ^ "European Biotech Company Biocell Center Opens First U.S. Facility for Preservation of Amniotic Stem Cells in Medford, Massachusetts". Reuters. 2009-10-22. http://www.reuters.com/article/pressRelease/idUS166682+22-Oct-2009+PRN20091022. Retrieved 2010-03-14.
- ^ "Europe's Biocell Center opens Medford office – Daily Business Update – The Boston Globe". Boston.com. 2009-10-22. http://www.boston.com/business/ticker/2009/10/europes_biocell.html. Retrieved 2010-03-14.
- ^ Track, Inside (2009-10-22). "The Ticker". BostonHerald.com. http://www.bostonherald.com/business/general/view/20091022the_ticker/. Retrieved 2010-03-14.
- ^ Biocell opens amniotic stem cell bank
- ^ "News » World’s First Amniotic Stem Cell Bank Opens In Medford". wbur.org. http://www.wbur.org/2009/10/22/stem-cell-bank. Retrieved 2010-03-14.
- ^ "Biocell Center Corporation Partners with New England's Largest Community-Based Hospital Network to Offer a Unique... – MEDFORD, Mass., March 8 /PRNewswire/". Massachusetts: Prnewswire.com. http://www.prnewswire.com/news-releases/biocell-center-corporation-partners-with-new-englands-largest-community-based-hospital-network-to-offer-a-unique-service-in-amniotic-fluid-stem-cell-preservation-86848157.html. Retrieved 2010-03-14.
- ^ a b c "Making human embryonic stem cells". The Economist. 2007-11-22. http://www.economist.com/science/displaystory.cfm?story_id=10170972.
- ^ Madeleine Brand, Joe Palca and Alex Cohen (2007-11-20). "Skin Cells Can Become Embryonic Stem Cells". National Public Radio. http://www.npr.org/templates/story/story.php?storyId=16466265.
- ^ "Breakthrough Set to Radically Change Stem Cell Debate". News Hour with Jim Lehrer. 2007-11-20. http://www.pbs.org/newshour/bb/science/july-dec07/stemcells_11-20.html.
- ^ "His inspiration comes from the research by Prof Shinya Yamanaka at Kyoto University, which suggests a way to create human embryo stem cells without the need for human eggs, which are in extremely short supply, and without the need to create and destroy human cloned embryos, which is bitterly opposed by the pro life movement."Roger Highfield (2007-11-16). "Dolly creator Prof Ian Wilmut shuns cloning". London: The Telegraph. http://www.telegraph.co.uk/science/science-news/3314696/Dolly-creator-Prof-Ian-Wilmut-shuns-cloning.html.
- ^ http://www.newsdaily.com/stories/tre6604si-us-stemcells-frozen/
- ^ Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B (2007). "Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins". Blood 109 (12): 5494–501. doi:10.1182/blood-2006-11-055921. PMID 17332245.
- ^ Xie T, Spradling A (1998). "decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary". Cell 94 (2): 251–60. doi:10.1016/S0092-8674(00)81424-5. PMID 9695953.
- ^ Song X, Zhu C, Doan C, Xie T (2002). "Germline stem cells anchored by adherens junctions in the Drosophila ovary niches". Science 296 (5574): 1855–7. doi:10.1126/science.1069871. PMID 12052957.
- ^ Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010-02-25). "Direct conversion of fibroblasts to functional neurons by defined factors". Nature 463 (7284): 1035–41. doi:10.1038/nature08797. PMC 2829121. PMID 20107439. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2829121. Lay summary.
- ^ Diabetes, rheumatoid arthritis, Parkinson's, Alzheimer's disease, osteoarthritis:
- Cell Basics: What are the potential uses of human stem cells and the obstacles that must be overcome before these potential uses will be realized?. In Stem Cell Information World Wide Web site. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services, 2009. cited Sunday, April 26, 2009
- Stem Cells Tapped to Replenish Organs thescientist.com, Nov 2000. By Douglas Steinberg
- ISRAEL21c: Israeli scientists reverse brain birth defects using stem cells December 25, 2008. (Researchers from the Hebrew University of Jerusalem-Hadassah Medical led by Prof. Joseph Yanai)
- Kang KS, Kim SW, Oh YH, et al. (2005). "A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study". Cytotherapy 7 (4): 368–73. doi:10.1080/14653240500238160. PMID 16162459.
- Strauer BE, Schannwell CM, Brehm M (April 2009). "Therapeutic potentials of stem cells in cardiac diseases". Minerva Cardioangiol 57 (2): 249–67. PMID 19274033.
- Stem Cells Tapped to Replenish Organs thescientist.com, Nov 2000. By Douglas Steinberg
- Hair Cloning Nears Reality as Baldness Cure WebMD November 2004
- Yen AH, Sharpe PT (January 2008). "Stem cells and tooth tissue engineering". Cell Tissue Res. 331 (1): 359–72. doi:10.1007/s00441-007-0467-6. PMID 17938970.
- Drs. Gearhart and Kerr of Johns Hopkins University. April 4, 2001 edition of JAMA (Vol. 285, 1691–1693)
- Querida Anderson (2008-06-15). "Osiris Trumpets Its Adult Stem Cell Product". Genetic Engineering & Biotechnology News (Mary Ann Liebert, Inc.): p. 13. http://www.genengnews.com/articles/chitem.aspx?aid=2508. Retrieved 2008-07-06. "(subtitle) Procymal is being developed in many indications, GvHD being the most advanced"
- Gurtner GC, Callaghan MJ, Longaker MT. (2007). "Progress and potential for regenerative medicine". Annu. Rev. Med 58 (1): 299–312. doi:10.1146/annurev.med.58.082405.095329. PMID 17076602.
- ^ Gahrton G, Björkstrand B (2000). "Progress in haematopoietic stem cell transplantation for multiple myeloma". J Intern Med 248 (3): 185–201. doi:10.1046/j.1365-2796.2000.00706.x. PMID 10971785.
- ^ Lindvall O (2003). "Stem cells for cell therapy in Parkinson's disease". Pharmacol Res 47 (4): 279–87. doi:10.1016/S1043-6618(03)00037-9. PMID 12644384.
- ^ Goldman S, Windrem M (2006). "Cell replacement therapy in neurological disease". Philos Trans R Soc Lond B Biol Sci 361 (1473): 1463–75. doi:10.1098/rstb.2006.1886. PMC 1664668. PMID 16939969. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1664668.
- ^ "Stem-cell therapy: Promise and reality." Consumer Reports on Health 17.6 (2005): 8–9. Academic Search Premier. EBSCO. Web. 5 Apr. 2010.
- ^ Wade N (2006-08-14). "Some Scientists See Shift in Stem Cell Hopes". New York Times. http://www.nytimes.com/2006/08/14/washington/14stem.html?_r=1. Retrieved 2006-12-28.
- ^ Regalado, Antonio, David P. Hamilton (July 2006). "How a University's Patents May Limit Stem-Cell Researcher." The Wall Street Journal. Retrieved on July 24, 2006.
- ^ Kintisch, Eli (July 18, 2006) "Groups Target Stem Cell Patents." ScienceNOW Daily News. Retrieved August 15, 2006.
- ^ Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (November 1998). "Embryonic stem cell lines derived from human blastocysts". Science (New York) 282 (5391): 1145–7. doi:10.1126/science.282.5391.1145. PMID 9804556.
- ^ Cibelli JB, Lanza RP, West MD, Ezzell C (November 2001). "The first human cloned embryo". Scientific American. http://www.scientificamerican.com/article.cfm?id=the-first-human-cloned-em.
- ^ Shostak S (2006). "(Re)defining stem cells". Bioessays 28 (3): 301–8. doi:10.1002/bies.20376. PMID 16479584.
- ^ Keirstead, HS; Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. (May 2005). "Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury.". The Journal of Neuroscience 25 (19): 4694–4705. doi:10.1523/JNEUROSCI.0311-05.2005. PMID 15888645. http://www.jneurosci.org/content/25/19/4694.full.pdf+html. Retrieved 23 July 2011.
- ^ Takahashi K, Yamanaka S (Aug 2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell 126 (4): 663–76. doi:10.1016/j.cell.2006.07.024. PMID 16904174.
- ^ "Good news for alcoholics". Discover Magazine. March 2007. http://discovermagazine.com/2007/mar/good-news-for-alcoholics. Retrieved 2010-02-28.
- ^ Ross, ShãN (2006-10-31). "First liver grown from stem cells offers hope for transplant patients". Edinburgh: The Scotsman. Archived from the original on 2007-02-03. http://web.archive.org/web/20070203010452/http://news.scotsman.com/health.cfm?id=1608072006.
- ^ De Coppi P, Bartsch G, Siddiqui MM, et al. (January 2007). "Isolation of amniotic stem cell lines with potential for therapy". Nat Biotechnol 25 (1): 100–6. doi:10.1038/nbt1274. PMID 17206138. http://www.nature.com/nbt/journal/v25/n1/abs/nbt1274.html.
- ^ Karen Kaplan (8 January 2007). "Easy stem-cell source sparks interest: Researchers find amniotic fluid offers advantages". Boston Globe. http://www.boston.com/news/nation/articles/2007/01/08/easy_stem_cell_source_sparks_interest/.
- ^ Cyranoski D (2007). "Simple switch turns cells embryonic". Nature 447 (7145): 618–9. doi:10.1038/447618a. PMID 17554270.
- ^ Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP (2007). "Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling". Hum Reprod 22 (8): 2232–42. doi:10.1093/humrep/dem136. PMID 17562675.
- ^ "The Nobel prize in physiology or medicine 2007". Nobelprize.org. http://nobelprize.org/nobel_prizes/medicine/laureates/2007/index.html. Retrieved 8 October 2007.
- ^ Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (November 2007). "Induction of pluripotent stem cells from adult human fibroblasts by defined factors" (PDF). Cell 131 (5): 861–72. doi:10.1016/j.cell.2007.11.019. PMID 18035408. http://images.cell.com/images/Edimages/Cell/IEPs/3661.pdf.
- ^ Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (December 2007). "Induced pluripotent stem cell lines derived from human somatic cells". Science 318 (5858): 1917–20. doi:10.1126/science.1151526. PMID 18029452. http://www.sciencemag.org/cgi/content/abstract/1151526.
- ^ Chung et al.; Klimanskaya, I; Becker, S; Li, T; Maserati, M; Lu, SJ; Zdravkovic, T; Ilic, D et al. (2008). "Human embryonic stem cell lines generated without embryo destruction". Cell Stem Cell 2 (2): 113. doi:10.1016/j.stem.2007.12.013. PMID 18371431. http://www.cell.com/cell-stem-cell/abstract/S1934-5909(07)00330-X.
- ^ French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH (2008-01-17). "Development of human cloned blastocysts following somatic cell nuclear transfer (SCNT) with adult fibroblasts". Stem Cells Express 26 (2): 485. doi:10.1634/stemcells.2007-0252. PMID 18202077. Archived from the original on 2008-06-25. http://web.archive.org/web/20080625032536/http://stemcells.alphamedpress.org/cgi/reprint/2007-0252v1.pdf.[dead link]
- ^ Aoi T, Yae K, Nakagawa M, et al. (August 2008). "Generation of pluripotent stem cells from adult mouse liver and stomach cells". Science 321 (5889): 699–702. doi:10.1126/science.1154884. PMID 18276851.
- ^ Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (2008). "Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells". Pain Physician 11 (3): 343–53. ISSN 1533-3159. PMID 18523506. http://www.painphysicianjournal.com/linkout_vw.php?issn=1533-3159&vol=11&page=343.
- ^ Conrad S, Renninger M, Hennenlotter J, et al. (November 2008). "Generation of pluripotent stem cells from adult human testis". Nature 456 (7220): 344–9. doi:10.1038/nature07404. PMID 18849962.
- ^ Baker M (October 2008). "Embryonic-like stem cells from a single human hair". Nature Reports Stem Cells. doi:10.1038/stemcells.2008.142. http://www.nature.com/stemcells/2008/0810/081030/full/stemcells.2008.142.html.
- ^ Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A (2009-03-01). "piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells". Nature 458 (7239): 766. doi:10.1038/nature07863. PMID 19252478.
- ^ "Canadians make stem cell breakthrough". March 1, 2009. http://www.ctv.ca/servlet/ArticleNews/story/CTVNews/20090227/stem_cells_090228/20090301?hub=TopStories. Retrieved March 1, 2009.
- ^ "Researchers find new method for turning adult cells into stem cells". Canadian Press. Amherst Daily News. 2009-01-03. http://www.amherstdaily.com/index.cfm?sid=227086&sc=510. Retrieved 2010-02-28.
- ^ Ian Sample (2009-03-01). "Scientists' stem cell breakthrough ends ethical dilemma". London: The Guardian. http://www.guardian.co.uk/science/2009/mar/01/stem-cells-breakthrough. Retrieved 2009-03-03.
- ^ Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K (2009-03-01). "Virus-free induction of pluripotency and subsequent excision of reprogramming factors". Nature 458 (7239): 771. doi:10.1038/nature07864. PMC 2667910. PMID 19252477. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2667910.
- ^ Lee ASJ, Kahatapitiya P, Kramer B, Joya JE, Hook J, Liu R, Schevzov G, Alexander IE, McCowage G, Montarras D, Gunning PW, Hardeman EC (2009). "Methylguanine DNA methyltransferase-mediated drug resistance-based selective enrichment and engraftment of transplanted stem cells in skeletal muscle". Stem Cells 27 (5): 1098–1108. doi:10.1002/stem.28. PMID 19415780. http://www3.interscience.wiley.com/journal/121683666/abstract.
- ^ Sample I (1 March 2009). "Scientists' stem cell breakthrough ends ethical dilemma". London: The Guardian. http://www.guardian.co.uk/science/2009/mar/01/stem-cells-breakthrough.
- ^ Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS (27 May 2009). "Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins". Cell Stem Cell 4 (6): 472–6. doi:10.1016/j.stem.2009.05.005. PMC 2705327. PMID 19481515. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2705327. Lay summary. (cited in lay summary, not read)
- ^ "First trial of embryonic stem cells in humans". BBC News. 2010-10-11. http://www.bbc.co.uk/news/health-11517680.
- ^ Ishikawa K, Toyama-Sorimachi N, Nakada K, Morimoto M, Imanishi H, Yoshizaki M, Sasawatari S, Niikura M, Takenaga K, Yonekawa H, Hayashi J (25 October 2010). "The innate immune system in host mice targets cells with allogenic mitochondrial DNA". J Exp Med. 207 (11): 2297–305. doi:10.1084/jem.20092296. PMC 2964578. PMID 20937705. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2964578.
- ^ Israeli scientist leads breakthrough stem cell research on endangered species
- 2011: American scientists produced the first human stem cells.
External links
- General
- Stem Cell Basics
- Nature Reports Stem Cells: Introductory material, research advances and debates concerning stem cell research.
- Understanding Stem Cells: A View of the Science and Issues from the National Academies
- Scientific American Magazine (June 2004 Issue) The Stem Cell Challenge
- Scientific American Magazine (July 2006 Issue) Stem Cells: The Real Culprits in Cancer?
- Ethics of Stem Cell Research entry by Andrew Siegel in the Stanford Encyclopedia of Philosophy
- Isolation of amniotic stem cell lines with potential for therapy
- Children's Hospital Stem Cell Research
- Stem Cell Research and Industry Directory
- Corneal endothelial and epithelial stem cell research and application
- Stem Cell Consumer Progess and Research
- Peer-reviewed journals
- Cytotherapy
- Cloning and Stem Cells
- Journal of Stem Cells and Regenerative Medicine
- Stem Cells and Development
- Regenerative Medicine
- Stem Cell Research
Wound healing Blood vessels Other Cellular differentiation: Stem cells / progenitor cell Sources/types Cell potency Totipotent (Zygote, Spore, Morula) · Pluripotent (Embryonic stem cell, Callus) · Multipotent (Progenitor cell: Endothelial stem cell, Hematopoietic stem cell, Mesenchymal stem cell, Neural stem cell) · Unipotent (Precursor cell)Related articles Stem cell treatments · Stem cell controversy · Stem cell line · Stem cell laws · Stem cell laws and policy in the United StatesCategories:
Wikimedia Foundation. 2010.