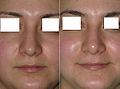
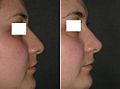
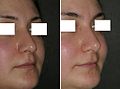
- Rhinoplasty
-
For the album by Primus, see Rhinoplasty (album).
Rhinoplasty Intervention 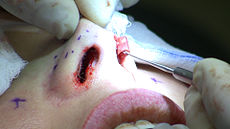
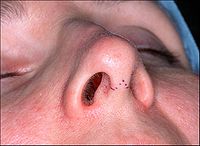
Rhinoplasty: The lower lateral cartilage (greater alar cartilage) exposed for plastic modification via the left nostril.ICD-9-CM 21.87 MeSH D012225 Rhinoplasty (Greek: ῥίς rhis, nose + πλάσσειν plassein, to shape), also nose job, is a plastic surgery procedure for correcting and reconstructing the form, restoring the functions, and aesthetically enhancing the nose, by resolving nasal trauma (blunt, penetrating, blast), congenital defect, respiratory impediment, and a failed primary rhinoplasty. In the surgeries — closed rhinoplasty and open rhinoplasty — an otolaryngologist (ear, nose, and throat specialist), a maxillofacial surgeon (jaw, face, and neck specialist), or a plastic surgeon, creates a functional, aesthetic, and facially proportionate nose by separating the nasal skin and the soft tissues from the osseo-cartilaginous nasal framework, correcting them as required for form and function, suturing the incisions, and applying a stent to immobilize the corrected (new) nose to ensure the proper healing of the surgical cuts.[1]
Contents
History
- Surgical rhinoplasty
- Antiquity
Rhinoplasty (reconstructive nose surgery) was first developed in ancient India, by the ayurvedic physician Sushruta (ca. 800 BC), who described reconstruction of the nose in the Sushruta samhita (ca. 500 BC), his medico–surgical compendium. The physician Sushruta and his medical students developed and applied plastic surgical techniques for reconstructing noses, genitalia, earlobes, et cetera, that were amputated as religious, criminal, or military punishment. Sushruta also developed the otoplastic technique for reconstructing an earlobe with skin from the cheek, and the forehead flap rhinoplasty procedure that remains contemporary plastic surgical practice. In the Sushruta samhita compendium, the physician Sushruta describes the (modern) free-graft Indian rhinoplasty as the Nasikasandhana, wherein:
The portion of the nose to be covered should be first measured with a leaf. Then, a piece of skin of the required size should be dissected from the living skin of the cheek, and turned back to cover the nose, keeping a small pedicle attached to the cheek. The part of the nose to which the skin is to be attached should be made raw, by cutting the nasal stump with a knife. The physician then should place the skin on the nose and stitch the two parts swiftly, keeping the skin properly elevated, by inserting two tubes of eranda (the castor-oil plant) in the position of the nostrils, so that the new nose has proper shape. The skin thus properly adjusted, it should then be sprinkled with a powder of liquorice, red sandal-wood, and barberry plant. Finally, it should be covered with cotton, and clean sesame oil should be continually applied. When the skin has united and granulated, if the nose is too short or too long, the middle of the flap should be divided, and an endeavour made to enlarge or shorten it. (Sushruta samhita 1.16)[2]
- Classical antiquity
During the Roman Empire (27 BC – AD 476) the encyclopaedist Aulus Cornelius Celsus (ca. 25 BC – AD 50) published the 8-tome De Medicina (On Medicine, ca. AD 14), which described plastic surgery techniques and procedures for the correction and the reconstruction of the lips, the ears, the nose, et cetera, and for the amputation of diseased and damaged parts of the human body.[3]
At the Byzantine Roman court of the Emperor Julian the Apostate (AD 331–363), the royal physician Oribasius (ca. AD 320–400) published the 70-volume Synagogue Medicae (Medical Compilations, AD 4th c.), which described facial-defect reconstructions that featured loose sutures that permitted a surgical wound to heal without distorting the facial flesh; how to clean the bone exposed in a wound; debridement, how to remove damaged tissue to forestall infection and so accelerate healing of the wound; and how to use autologous skin flaps to repair damaged cheeks, eyebrows, lips, and nose, to restore the patient’s normal visage.
- The Dark Ages
Nonetheless, during the centuries of the European Dark Ages (AD 5th – 15th centuries) that followed the Imperial Roman collapse (AD 476), the fifth-century BC Asian plastic surgery knowledge of the Sushruta samhita went unknown to the West until the tenth century AD, with the publication, in Old English, of the Anglo-Saxon physician’s manual Bald's Leechbook (ca. AD 920) describing the plastic repair of a cleft lip; as a medical compendium, the Leechbook is notable for categorizing ailments and treatments as internal medicine and as external medicine, for providing herbal medical remedies, and for providing supernatural incantations (prayers), when required.
In the eleventh century, at Damascus, the Arab physician Ibn Abi Usaibia (1203–1270) translated the Sushruta samhita from Sanskrit to Arabic. In due course, Sushruta’s medical compendium travelled from Arabia to Persia to Egypt, and, by the fifteenth century, Western European medicine had encountered it as the medical atlas Cerrahiyet-ul Haniye (Imperial Surgery, 15th c.), by Şerafeddin Sabuncuoğlu (1385–1468); among its surgical techniques featured a breast reduction procedure.[4][5]
- 16th century
In Italy, Gasparo Tagliacozzi (1546–1599), professor of surgery and anatomy at the University of Bologna, published Curtorum Chirurgia Per Insitionem (The Surgery of Defects by Implantations, 1597), a technico–procedural manual for the surgical repair and reconstruction of facial wounds in soldiers. The illustrations featured a re-attachment rhinoplasty using a biceps muscle pedicle flap; the graft attached at 3-weeks post-procedure; which, at 2-weeks post-attachment, the surgeon then shaped into a nose.[6]
- 18th century;
In time, the fifth-century BC Indian rhinoplasty technique — featuring a free-flap graft — was (re) discovered by Western medicine in the eighteenth century, during the Third Anglo–Mysore War (1789–1792) of colonial annexation, by the British against Tipu Sultan, when the East India Company surgeons Thomas Cruso and James Findlay witnessed Indian rhinoplasty procedures at the British Residency in Poona. In the English-language Madras Gazette, the surgeons published photographs of the rhinoplasty procedure and its nasal reconstruction outcomes; later, in the October 1794 issue of the Gentleman's Magazine of London, the doctors Cruso and Findlay published an illustrated report describing a forehead pedicle-flap rhinoplasty that was a technical variant of the free-flap graft technique that Sushruta had described some twenty-three centuries earlier:
A thin plate of wax is fitted to the stump of the nose, so as to make a nose of good appearance; it is then flattened and laid on the forehead. A line is drawn around the wax, which is then of no further use, and the operator then dissects off as much skin [the flap] as [the wax plate] had covered, leaving undivided a small slip [the flap-pedicle] between the eyes. This slip preserves the blood circulation till a union has taken place between the new and the old parts. The cicatrix of the stump of the nose is next pared off, and, immediately behind the new part, an incision is made through the skin, which passes around both alae, and goes along the upper lip. The skin, now brought down from the forehead, and being twisted half-around, is inserted into this incision, so that a nose is formed with a double hold above, and with its alae and septum below, fixed in the incision. A little Terra Japonica (pale-catechu) is softened with water, and after being spread on slips of cloth, five or six of these are placed over each other to secure the joining. No other dressing, but this cement, is used for four days. It is then removed, and cloths dipped in ghee are applied. The connecting slip of skin is divided at about the twentieth day, when a little more dissection is necessary to improve the appearance of the new nose. For five or six days after the operation, the patient is made to lie on his back, and on the tenth day, bits of soft cloth are put into the nostrils to keep them sufficiently open. This operation is always successful. The artificial nose is secured and looks nearly as well as the natural nose, nor is the scar on the forehead very observable after a length of time. (Gentleman’s Magazine of London, October 1794)[7]
- 19th century
Pre-dating the Indian Sushruta samhita medical compendium is the Ebers Papyrus (ca. 1550 BC), an Ancient Egyptian medical papyrus that describes rhinoplasty as the plastic surgical operation for reconstructing a nose destroyed by rhinectomy, such a mutilation was inflicted as a criminal, religious, political, and military punishment in that time and culture.[8] In the event, the Indian rhinoplasty technique perdured in nineteenth-century Western European medicine; in Great Britain, Joseph Constantine Carpue (1764–1846) published the Account of Two Successful Operations for Restoring a Lost Nose (1815), which described two rhinoplasties: the reconstruction of a battle-wounded nose, and the repair of an arsenic-damaged nose. (cf. Carpue’s operation)[2][9]
In Germany, rhinoplastic technique was refined by surgeons such as the Berlin University professor of surgery Karl Ferdinand von Gräfe (1787–1840), who published Rhinoplastik (Rebuilding the Nose, 1818) wherein he described fifty-five (55) historical plastic surgery procedures (Indian rhinoplasty, Italian rhinoplasty, etc.), and his technically innovative free-graft nasal reconstruction (with a tissue-flap harvested from the patient’s arm), and surgical approaches to eyelid, cleft lip, and cleft palate corrections. Dr. von Gräfe’s protégé, the medical and surgical polymath Johann Friedrich Dieffenbach (1794–1847), who was among the first surgeons to anaesthetize the patient before performing the nose surgery, published Die Operative Chirurgie (Operative Surgery, 1845), which became a foundational medical and plastic surgical text. (see strabismus, torticollis) Moreover, the Prussian Jacques Joseph (1865–1934) published Nasenplastik und sonstige Gesichtsplastik (Rhinoplasty and other Facial Plastic Surgeries, 1928), which described refined surgical techniques for performing nose-reduction rhinoplasty via internal incisions.[10]
In the United States, in 1887, the otolaryngologist John Orlando Roe (1848–1915) performed the first, modern endonasal rhinoplasty (closed rhinoplasty), about which he reported in the article The Deformity Termed “Pug Nose” and its Correction, by a Simple Operation (1887), and about his management of saddle nose deformities.[11][12]
- 20th century
In the early twentieth century, Freer, in 1902, and Killian, in 1904, respectively pioneered the submucous resection septoplasty (SMR) procedure for correcting a deviated septum; they raised mucoperichondrial tissue flaps, and resected the cartilaginous and bony septum (including the vomer bone and the perpendicular plate of the ethmoid bone), maintaining septal support with a 1.0-cm margin at the dorsum and a 1.0-cm margin at the caudad, for which innovations the technique became the foundational, standard septoplastic procedure. In 1921, A. Rethi introduced the open rhinoplasty approach featuring an incision to the columella to facilitate modifying the tip of the nose.[13] In 1929, Peer and Metzenbaum performed the first manipulation of the caudal septum, where it originates and projects from the forehead. In 1947, Maurice H. Cottle (1898–1981) endonasally resolved a septal deviation with a minimalist hemitransfixion incision, which conserved the septum; thus, he advocated for the practical primacy of the closed rhinoplasty approach.[8] In 1957, A. Sercer advocated the “decortication of the nose” (Dekortication des Nase) technique which featured a columellar-incision open rhinoplasty that allowed greater access to the nasal cavity and to the nasal septum.
Nonetheless, at mid–twentieth century, despite such refinement of the open rhinoplasty approach, the endonasal rhinoplasty was the usual approach to nose surgery — until the 1970s, when Padovan presented his technical refinements, advocating the open rhinoplasty approach; he was seconded by Wilfred S. Goodman in the later 1970s, and by Jack P. Gunter in the 1990s.[14][15] Goodman impelled technical and procedural progress with the article External Approach to Rhinoplasty (1973), which reported his technical refinements and popularized the open rhinoplasty approach.[16] In 1982, Jack Anderson reported his refinements of nose surgery technique in the article Open Rhinoplasty: An Assessment (1982).[17] During the 1970s, the principal application of open rhinoplasty was to the first-time rhinoplasty patient (i.e. a primary rhinoplasty), not as a revision surgery (i.e. a secondary rhinoplasty) to correct a failed nose surgery. In 1987, in the article External Approach for Secondary Rhinoplasty (1987), Jack P. Gunter reported the technical effectiveness of the open rhinoplasty approach for performing a secondary rhinoplasty; his improved techniques advanced the management of a failed nose surgery.[18]
Hence does contemporary rhinoplastic praxis derive from the primeval (ca. 600 BC) Indian rhinoplasty (nasal reconstruction via an autologous forehead-skin flap) and its technical variants: Carpue’s operation, the Italian rhinoplasty (pedicle-flap reconstruction, aka the Tagliocotian rhinoplasty); and the closed-approach endonasal rhinoplasty, featuring exclusively internal incisions that allow the plastic surgeon to palpate (feel) the corrections being effected to the nose.[19]
- Non-surgical rhinoplasty
- 19th century
Non-surgical rhinoplasty originated at the turn of the nineteenth century, when the New York City neurologist James Leonard Corning (1855–1923) and the Viennese physician Robert Gersuny (1844–1924) pioneered the technique of using liquid paraffin to elevate the collapsed nasal dorsum that characterizes the saddle nose deformity. Yet, despite its corrective efficacy, liquid paraffin proved biologically harmful, and its medical use as a nasal soft-tissue filler, was abandoned because it caused paraffinoma (wax cancer), a serious medical complication.
- 20th century
During the 1960s, soft-tissue fillers of medical-grade silicone gel were introduced to the rhinoplastic armamentarium, however, like liquid paraffin, silicone gels also proved biologically harmful, because they caused medical complications such as the corrective soft-tissue filler migrating from the nasal tissues to elsewhere in the patient’s face and body, and the development of ulcers and granulomas.[20] To minimize the risk of these medical complications for the patient, practitioners such as D.S. Orentreich advocated effecting the non-surgical correction by “microdroplet technique”, minute doses of silicone injected in the course of multiple correction sessions.[21]
- 21st century
Contemporary non-surgical rhinoplasty was established with the Australian study Rhinoplasty Using Injectable Polyacrylamide Gel — A Patient Study (2005), wherein Dr. Andrew Tuan-anh Le reported successful corrective outcomes using the soft-tissue filler polyacrylamide gel (PAAG), a hydrophilic colloid injected to the tissues of the nasal defect or deformity in order to (re)create a functional and aesthetically proportionate nose for the patient.[22][23] Among the subcutaneous soft-tissue filler agents employed to achieve non-surgical rhinoplasty are: injectable silicone, calcium hydroxyapatite, hyaluronic acid, collagen (human, bovine, and porcine), and polymethylmethacrylate. Possible complications include infection, hematoma, discomfort, anatomic asymmetry, foreign body reaction (rejection) or granuloma, or both, over-correction, under-correction, intravascular injection (with potential for thromboembolism), adverse medication reaction (anaphylaxis), allergic reaction, the need for additional “touch up” injections of the tissue filler, palpability, visibility, distortion with muscular contraction, the need for secondary revisions, and the physician’s inability to guarantee a specific cosmetic result to the patient.
Anatomy of the human nose
- Embryologic development
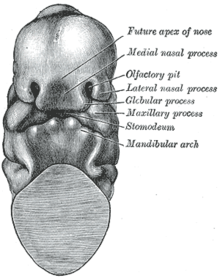
At four (4) weeks of gestational development, the neural crest cells (the precursors of the nose) begin their caudad migration (from the posterior) towards the midface. Two symmetrical nasal placodes (the future olfactory epithelium) develop inferiorly, which the nasal pits then divide into the medial and the lateral nasal processes (the future upper lip and nose). The medial processes then form the septum, the philtrum, and the premaxilla of the nose; the lateral processes form the sides of the nose; and the mouth forms from the stomodeum (the anterior ectodermal portion of the alimentary tract), which is inferior to the nasal complex.
A nasobuccal membrane separates the mouth from the nose; respectively, the inferior oral cavity (the mouth) and the superior nasal cavity (the nose). As the olfactory pits deepen, said development forms the choanae, the two openings that connect the nasal cavity and the nasopharynx (upper part of the pharynx that is continuous with the nasal passages). Initially, primitive-form choanae develop, which then further develop into the secondary, permanent choanae.
At ten (10) weeks of gestation, the cells differentiate into muscle, cartilage, and bone. If this important, early facial embryogenesis fails, it might result in anomalies such as choanal atresia (absent or closed passage), medial nasal clefts (fissures), or lateral nasal clefts, nasal aplasia (faulty or incomplete development), and polyrrhinia (double nose).[24]
This normal, human embryologic development is exceptionally important — because the newborn infant breathes through his or her nose during the first 6 weeks of life — thus, when a child is afflicted with bilateral choanal atresia, the blockage of the posterior nasal passage, either by abnormal bony tissue or by abnormal soft tissue, emergency remedial action is required to ensure that the child can breathe.[25]
- The structures of the nose
For plastic surgical correction, the structural anatomy of the nose comprehends: A. the nasal soft tissues; B. the aesthetic subunits and segments; C. the blood supply arteries and veins; D. the nasal lymphatic system; E. the facial and nasal nerves; F. the nasal bones; and G. the nasal cartilages.
- A. The nasal soft tissues
- Nasal skin — Like the underlying bone-and-cartilage (osseo-cartilaginous) support framework of the nose, the external skin is divided into vertical thirds (anatomic sections); from the glabella (the space between the eyebrows), to the bridge, to the tip, for corrective plastic surgery, the nasal skin is anatomically considered, as the:
- Upper third section — the skin of the upper nose is thick, and relatively distensible (flexible and mobile), but then tapers, adhering tightly to the osseo-cartilaginous framework, and becomes the thinner skin of the dorsal section, the bridge of the nose.
- Middle third section — the skin overlying the bridge of the nose (mid-dorsal section) is the thinnest, least distensible, nasal skin, because it most adheres to the support framework.
- Lower third section — the skin of the lower nose is as thick as the skin of the upper nose, because it has more sebaceous glands, especially at the nasal tip.
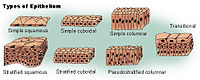
- Nasal lining — At the vestibule, the human nose is lined with a mucous membrane of squamous epithelium, which tissue then transitions to become columnar respiratory epithelium, a pseudo-stratified, ciliated (lash-like) tissue with abundant seromucinous glands, which maintains the nasal moisture and protects the respiratory tract from bacteriologic infection and foreign objects.
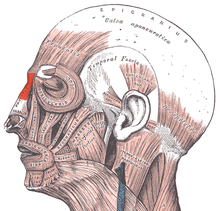
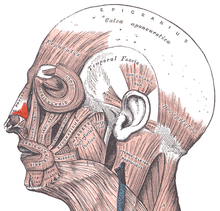
- Nasal muscles — The movements of the human nose are controlled by groups of facial and neck muscles that are set deep to the skin; they are in four (4) functional groups that are interconnected by the nasal superficial aponeurosis — the superficial musculoaponeurotic system (SMAS) — which is a sheet of dense, fibrous, collagenous connective tissue that covers, invests, and forms the terminations of the muscles.
- The movements of the nose are effected by
- the elevator muscle group — which includes the procerus muscle and the levator labii superioris alaeque nasi muscle.
- the depressor muscle group — which includes the alar nasalis muscle and the depressor septi nasi muscle.
- the compressor muscle group — which includes the transverse nasalis muscle.
- the dilator muscle group — which includes the dilator naris muscle that expands the nostrils; it is in two parts: (i) the dilator nasi anterior muscle, and (ii) the dilator nasi posterior muscle.
- B. Aesthetics of the nose — nasal subunits and nasal segments
To plan, map, and execute the surgical correction of a nasal defect or deformity, the structure of the external nose is divided into nine (9) aesthetic nasal subunits, and six (6) aesthetic nasal segments, which provide the plastic surgeon with the measures for determining the size, extent, and topographic locale of the nasal defect or deformity.
- The surgical nose as nine (9) aesthetic nasal subunits
- tip subunit
- columellar subunit
- right alar base subunit
- right alar wall subunit
- left alar wall subunit
- left alar base subunit
- dorsal subunit
- right dorsal wall subunit
- left dorsal wall subunit
In turn, the nine (9) aesthetic nasal subunits are configured as six (6) aesthetic nasal segments; each segment comprehends a nasal area greater than that comprehended by a nasal subunit.
- The surgical nose as six (6) aesthetic nasal segments
- the dorsal nasal segment
- the lateral nasal-wall segments
- the hemi-lobule segment
- the soft-tissue triangle segments
- the alar segments
- the columellar segment
Using the co-ordinates of the subunits and segments to determine the topographic location of the defect on the nose, the plastic surgeon plans, maps, and executes a rhinoplasty procedure. The unitary division of the nasal topography permits minimal, but precise, cutting, and maximal corrective-tissue coverage, to produce a functional nose of proportionate size, contour, and appearance for the patient. Hence, if more than 50 per cent of an aesthetic subunit is lost (damaged, defective, destroyed) the surgeon replaces the entire aesthetic segment, usually with a regional tissue graft, harvested from either the face or the head, or with a tissue graft harvested from elsewhere on the patient’s body.[26]
- C. Nasal blood supply — arteries and veins
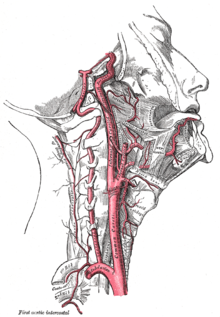
Like the face, the human nose is well vascularized with arteries and veins, and thus supplied with abundant blood. The principal arterial blood-vessel supply to the nose is two-fold: (i) branches from the internal carotid artery, the branch of the anterior ethmoid artery, the branch of the posterior ethmoid artery, which derive from the ophthalmic artery; (ii) branches from the external carotid artery, the sphenopalatine artery, the greater palatine artery, the superior labial artery, and the angular artery.
The external nose is supplied with blood by the facial artery, which becomes the angular artery that courses over the superomedial aspect of the nose. The sellar region (sella turcica, “Turkish chair”) and the dorsal region of the nose are supplied with blood by branches of the internal maxillary artery (infraorbital) and the ophthalmic arteries that derive from the internal common carotid artery system.
Internally, the lateral nasal wall is supplied with blood by the sphenopalatine artery (from behind and below) and by the anterior ethmoid artery and the posterior ethmoid artery (from above and behind). The nasal septum also is supplied with blood by the sphenopalatine artery, and by the anterior and posterior ethmoid arteries, with the additional circulatory contributions of the superior labial artery and of the greater palatine artery. These three (3) vascular supplies to the internal nose converge in the Kiesselbach plexus (the Little area), which is a region in the anteroinferior-third of the nasal septum, (in front and below). Furthermore, the nasal vein vascularisation of the nose generally follows the arterial pattern of nasal vascularisation. The nasal veins are biologically significant, because they have no vessel-valves, and because of their direct, circulatory communication to the sinus caverns, which makes possible the potential intracranial spreading of a bacterial infection of the nose. Hence, because of such an abundant nasal blood supply, tobacco smoking does therapeutically compromise post-operative healing.
- D. Lymphatic system of the nose
The pertinent nasal lymphatic system arises from the superficial mucosa, and drains posteriorly to the retropharyngeal nodes (in back), and anteriorly (in front), either to the upper deep cervical nodes (in the neck), or to the submandibular glands (in the lower jaw), or into both the nodes and the glands of the neck and the jaw.
- E. Nerves of the nose
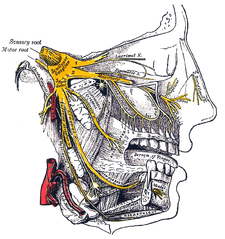
The sensations registered by the human nose derive from the first two (2) branches of cranial nerve V, the trigeminal nerve (nervus trigeminis). The nerve listings indicate the respective innervation (sensory distribution) of the trigeminal nerve branches within the nose, the face, and the upper jaw (maxilla).
- The indicated nerve serves the named anatomic facial and nasal regions
- Ophthalmic division innervation
- Lacrimal nerve — conveys sensation to the skin areas of the lateral orbital (eye socket) region, except for the lacrimal gland.
- Frontal nerve — conveys sensation to the skin areas of the forehead and the scalp.
- Supraorbital nerve — conveys sensation to the skin areas of the eyelids, the forehead, and the scalp.
- Supratrochlear nerve — conveys sensation to the medial region of the eyelid skin area, and the medial region of the forehead skin.
- Nasociliary nerve — conveys sensation to the skin area of the nose, and the mucous membrane of the anterior (front) nasal cavity.
- Anterior ethmoid nerve — conveys sensation in the anterior (front) half of the nasal cavity: (a) the internal areas of the ethmoid sinus and the frontal sinus; and (b) the external areas, from the nasal tip to the rhinion: the anterior tip of the terminal end of the nasal-bone suture.
- Posterior ethmoid nerve — serves the superior (upper) half of the nasal cavity, the sphenoids, and the ethmoids.
- Intratrochlear nerve — conveys sensation to the medial region of the eyelids, the palpebral conjunctiva, the nasion (nasolabial junction), and the bony dorsum.
The maxillary division innervation
- Maxillary nerve — conveys sensation to the upper jaw and the face.
- Infraorbital nerve — conveys sensation to the area from below the eye socket to the external nares (nostrils).
- Zygomatic nerve — through the zygomatic bone and the zygomatic arch, conveys sensation to the cheekbone areas.
- Superior posterior dental nerve — sensation in the teeth and the gums.
- Superior anterior dental nerve — mediates the sneeze reflex.
- Sphenopalatine nerve — divides into the lateral branch and the septal branch, and conveys sensation from the rear and the central regions of the nasal cavity.
The supply of parasympathetic nerves to the face and the upper jaw (maxilla) derives from the greater superficial petrosal (GSP) branch of cranial nerve VII, the facial nerve. The GSP nerve joins the deep petrosal nerve (of the sympathetic nervous system), derived from the carotid plexus, to form the vidian nerve (in the vidian canal) that traverses the pterygopalatine ganglion (an autonomic ganglion of the maxillary nerve), wherein only the parasympathetic nerves form synapses, which serve the lacrimal gland and the glands of the nose and of the palate, via the (upper jaw) maxillary division of cranial nerve V, the trigeminal nerve.
- F. Bony anatomy of the nose
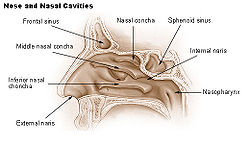
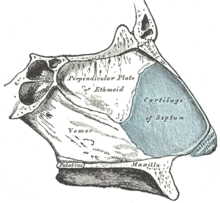
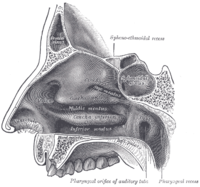
In the upper portion of the nose, the paired nasal bones attach to the frontal bone. Above and to the side (superolaterally), the paired nasal bones connect to the lacrimal bones, and below and to the side (inferolaterally), they attach to the ascending processes of the maxilla (upper jaw). Above and to the back (posterosuperiorly), the bony nasal septum is composed of the perpendicular plate of the ethmoid bone. The vomer bone lies below and to the back (posteroinferiorly), and partially forms the choanal opening into the nasopharynx, (the upper portion of the pharynx that is continuous with the nasal passages). The floor of the nose comprises the premaxilla bone and the palatine bone, the roof of the mouth.
The nasal septum is composed of the quadrangular cartilage, the vomer bone (the perpendicular plate of the ethmoid bone), aspects of the premaxilla, and the palatine bones. Each lateral nasal wall contains three pairs of turbinates (nasal conchae), which are small, thin, shell-form bones: (i) the superior concha, (ii) the middle concha, and (iii) the inferior concha, which are the bony framework of the turbinates. Lateral to the turbinates is the medial wall of the maxillary sinus. Inferior to the nasal conchae (turbinates) is the meatus space, with names that correspond to the turbinates, e.g. superior turbinate, superior meatus, et alii. The internal roof of the nose is composed by the horizontal, perforated cribriform plate (of the ethmoid bone) through which pass sensory filaments of the olfactory nerve (Cranial nerve I); finally, below and behind (posteroinferior) the cribriform plate, sloping down at an angle, is the bony face of the sphenoid sinus.
- G. The cartilaginous pyramid of the nose
The cartilaginous septum (septum nasi) extends from the nasal bones in the midline (above) to the bony septum in the midline (posteriorly), then down along the bony floor. The septum is quadrangular; the upper-half is flanked by two (2) triangular-to-trapezoidal cartilages: the upper lateral-cartilages, which are fused to the dorsal septum in the midline, and laterally attached, with loose ligaments, to the bony margin of the pyriform (pear-shaped) aperture, while the inferior ends of the upper lateral-cartilages are free (unattached). The internal area (angle), formed by the septum and upper lateral-cartilage, constitutes the internal valve of the nose; the sesamoid cartilages are adjacent to the upper lateral-cartilages in the fibroareolar connective tissue.
Beneath the upper lateral-cartilages lay the lower lateral-cartilages; the paired lower lateral-cartilages swing outwards, from medial attachments, to the caudal septum in the midline (the medial crura) to an intermediate crus (shank) area. Finally, the lower lateral-cartilages flare outwards, above and to the side (superolaterally), as the lateral crura; these cartilages are mobile, unlike the upper lateral cartilages. Furthermore, some persons present anatomical evidence of nasal scrolling — i.e. an outward curving of the lower borders of the upper lateral-cartilages, and an inward curving of the cephalic borders of the alar cartilages.
- The external nose
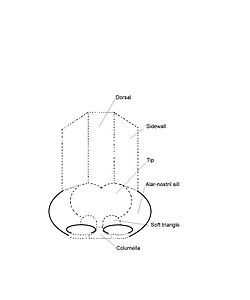
- External nasal anatomy
The form of the nasal subunits — the dorsum, the sidewalls, the lobule, the soft triangles, the alae, and the columella — are configured differently, according to the race and the ethnic group of the patient, thus the nasal physiognomies denominated as: African, platyrrhine (flat, wide nose); Asiatic, subplatyrrhine (low, wide nose); Caucasian, leptorrhine (narrow nose); and Hispanic, paraleptorrhine (narrow-sided nose). The respective external valve of each nose is variably dependent upon the size, shape, and strength of the lower lateral cartilage.[27]
- Internal nasal anatomy
In the midline of the nose, the septum is a composite (osseo-cartilaginous) structure that divides the nose into two (2) similar halves. The lateral nasal wall and the paranasal sinuses, the superior concha, the middle concha, and the inferior concha, form the corresponding passages, the superior meatus, the middle meatus, and the inferior meatus, on the lateral nasal wall. The superior meatus is the drainage area for the posterior ethmoid bone cells and the sphenoid sinus; the middle meatus provides drainage for the anterior ethmoid sinuses and for the maxillary and frontal sinuses; and the inferior meatus provides drainage for the nasolacrimal duct.
The internal nasal valve comprises the area bounded by the upper lateral-cartilage, the septum, the nasal floor, and the anterior head of the inferior turbinate. In the narrow (leptorrhine) nose, this is the narrowest portion of the nasal airway. Generally, this area requires an angle greater than 15 degrees for unobstructed breathing; for the correction of such narrowness, the width of the nasal valve can be increased with spreader grafts and flaring sutures.
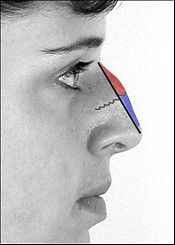
 Rhinoplasty. Nasal anatomy: The philtrum.
Rhinoplasty. Nasal anatomy: The philtrum.
- Nasal analysis
- angles and projection
The surgical management of nasal defects and deformities divides the nose into six (6) anatomic subunits: (i) the dorsum, (ii) the sidewalls (paired), (iii) the hemilobules (paired), (iv) the soft triangles (paired), (v) the alae (paired), and (vi) the columella. Surgical correction and reconstruction comprehend the entire anatomic subunit affected by the defect (wound) or deformity, thus, the entire subunit is corrected, especially when the resection (cutting) of the defect encompasses more than 50 per cent of the subunit. Aesthetically, the nose — from the nasion (the mid-point of the nasofrontal junction) to the columella-labial junction — ideally occupies one-third of the vertical dimension of the person’s face; and, from ala to ala, it ideally should occupy one-fifth of the horizontal dimension of the person’s face.[28]
The nasofrontal angle, between the frontal bone and the nasion usually is 120 degrees; the nasofrontal angle is more acute in the male face than in the female face. The nasofacial angle, the slope of the nose relative to the plane of the face, is approximately 30–40 degrees. The nasolabial angle, the slope between the columella and the philtrum, is approximately 90–95 degrees in the male face, and approximately 100–105 degrees in the female face. Therefore, when observing the nose in profile, the normal show of the columella (the height of the visible nasal aperture) is 2–mm; and the dorsum should be rectilinear (straight). When observed from below (worm’s-eye view), the alar base configures an isosceles triangle, with its apex at the infra-tip lobule, immediately beneath the tip of the nose. The facially proportionate projection of the nasal tip (the distance of the nose’s tip from the face) is determined with the Goode Method, wherein the projection of the nasal tip should be 55-60 per cent of the distance between the nasion (nasofrontal junction) and the tip-defining point. A columellar double break might be present, marking the transition between the intermediate crus of the lower-lateral cartilage and the medial crus.
The Goode Method determines the extension of the nose from the facial surface by comprehending the distance from the alar groove to the tip of the nose, and then relating that measurement (of nasal-tip projection) to the length of the nasal dorsum. The nasal projection measurement is obtained by delineating a right triangle with lines parting from the nasion (nasofrontal juncture) to the alar–facial–groove. Then, a second, perpendicular delineation, that traverses the tip-defining point, establishes the ratio of projection of the nasal tip; hence, the range of 0.55:1 to 0.60:1, is the ideal nasal-tip-to-nasal-length projection.[29]
 Rhinoplasty: the SIMON patient, Narcissus (1599), by Caravaggio (Michelangelo Merissa).
Rhinoplasty: the SIMON patient, Narcissus (1599), by Caravaggio (Michelangelo Merissa).
- Patient characteristics
- Surgical suitability
- the medical and mental histories of the patient
To determine the patient’s suitability for undergoing a rhinoplasty procedure, the surgeon clinically evaluates him or her with a complete medical history (anamnesis) to determine his or her physical and psychological health. The prospective patient must explain to the physician–surgeon the functional and aesthetic nasal problems that he or she suffers. The surgeon asks about the ailments’ symptoms and their duration, past surgical interventions, allergies, drugs use and drugs abuse (prescription and commercial medications), and a general medical history. Furthermore, additional to physical suitability is psychological suitability — the patient’s psychological motive for undergoing nose surgery is critical to the surgeon’s pre-operative evaluation of the patient. In the case of men, the surgeon must identify prospective patients presenting the mental traits denoted by the psychiatric acronym SIMON (single, immature, male, over-expectant, and narcissistic), which might indicate a man over-valuing the outcome of a rhinoplasty.[8]
The complete physical examination of the rhinoplasty patient determines if he or she is physically fit to undergo and tolerate the physiologic stresses of nose surgery. The examination comprehends every existing physical problem, and a consultation with an anaesthesiologist, if warranted by the patient’s medical data. Specific facial and nasal evaluations record the patient’s skin-type, existing surgical scars, and the symmetry and asymmetry of the aesthetic nasal subunits. The external and internal nasal examination concentrates upon the anatomic thirds of the nose — upper section, middle section, lower section — specifically noting their structures; the measures of the nasal angles (at which the external nose projects from the face); and the physical characteristics of the naso-facial bony and soft tissues. The internal examination evaluates the condition of the nasal septum, the internal and external nasal valves, the turbinates, and the nasal lining, paying especial attention to the structure and the form of the nasal dorsum and the tip of the nose.[8]
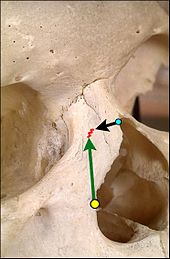
Furthermore, when warranted, specific tests — the mirror test, vasoconstriction examinations, and the Cottle maneuver — are included to the pre-operative evaluation of the prospective rhinoplasty patient. Established by Maurice H. Cottle (1898–1981), the Cottle maneuver is a principal diagnostic technique for detecting an internal nasal-valve disorder; whilst the patient gently inspires, the surgeon laterally pulls the patient’s cheek, thereby simulating the widening of the cross-sectional area of the corresponding internal nasal valve. If the maneuver notably facilitates the patient’s inspiration, that result is a positive Cottle sign — which generally indicates an airflow-correction to be surgically effected with an installed spreader-graft. Said correction will improve the internal angle of the nasal valve and thus allow unobstructed breathing. Nonetheless, the Cottle maneuver occasionally yields a false-positive Cottle sign, usually observed in the patient afflicted with alar collapse, and in the patient with a scarred nasal-valve region.[30]
Surgical rhinoplasty
- The approaches — open rhinoplasty and closed rhinoplasty
The plastic surgical correction of congenital and acquired abnormalities of the nose restores functional and aesthetic properties by the surgeon’s manipulations of the nasal skin, the subcutaneous (underlying) cartilage-and-bone support framework, and the mucous membrane lining. Technically, the plastic surgeon’s incisional approach classifies the nasal surgery either as an open rhinoplasty or as a closed rhinoplasty procedure. In open rhinoplasty, the surgeon makes a small, irregular incision to the columella, the fleshy, exterior-end of the nasal septum; this columellar incision is additional to the usual set of incisions for a nasal correction. In closed rhinoplasty, the surgeon performs every procedural incision endonasally (exclusively within the nose), and does not cut the columella.[8]
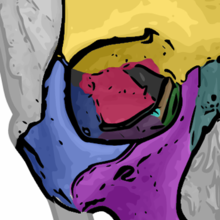 Rhinoplasty:
Rhinoplasty:
In relation to the nasal bone (teal green), seven (7) bones compose the orbit.
(1) the frontal bone (yellow)
(2) the lacrimal bone (green)
(3) the ethmoid bone (brown)
(4) the zygomatic bone (blue)
(5) the upper jaw maxillary bone (purple)
(6) the palatine bone (aqua)
(7) the sphenoid bone (red)- Procedural differences — except for the columellar incision, the technical and procedural approaches of open rhinoplasty and of closed rhinoplasty are similar; yet closed rhinoplasty procedure features:
- Reduced dissection (cutting) of the nasal tissues — no columellar incision
- Decreased potential for the excessive reduction (cutting) of the nasal-tip support
- Reduced post-operative edema
- Decreased visible scarring
- Decreased iatrogenic (inadvertent) damage to the nose, by the surgeon
- Increased availability for effecting in situ procedural and technical changes
- Palpation that allows the surgeon to feel the interior changes effected to the nose
- Shorter operating room time
- Quicker post-surgical recovery and convalescence for the patient[31]
- The “ethnic nose”
The open rhinoplasty approach affords the plastic surgeon the advantages of ease in securing the grafts (skin, cartilage, bone) and, most important, in seeing the nasal cartilages proper, and so make the appropriate diagnosis. This procedural aspect can be especially difficult in revision surgery, and in rhinoplastic corrections of the thick-skinned “ethnic nose” of the colored man or woman. The study, Ethnic Rhinoplasty: a Universal Preoperative Classification System for the Nasal Tip (2009), reports that a nasal-tip classification system, based upon skin thickness, has been proposed to aid the surgeon in determining if an open rhinoplasty or a closed rhinoplasty shall best correct the defect or deformity afflicting the patient’s nose.[32]
- Etiology
Etiologically, the open and closed approaches to rhinoplastic correction resolve: (i) nasal pathologies (diseases intrinsic and diseases extrinsic to the nose); (ii) an unsatisfactory aesthetic appearance (disproportion); (iii) a failed primary rhinoplasty; (iv) an obstructed airway; and (v) congenital nose defects and deformities.
- Congenital abnormalities such as
- Cleft lip and palate in combination; cleft lip (cheiloschisis) and cleft palate (palatoschisis), individually.
- Congenital nasal abnormalities
- Genetically derived ethnic-nose abnormalities
Acquired abnormalities such as:
- Allergic and vasomotor rhinitis — inflammations of the mucous membrane of the nose caused by an allergen, and caused by circulatory and nervous system disorders.
- Bites — animal and human
- Inflammatory conditions
- Nasal fractures
- Naso-orbito-ethmoidal fractures — damages to the nose and the eye-sockets; and damage to the bones and the walls of the nasal cavity; it is the ethmoid bone that separates the brain from the nose.
- Septal hematoma — a mass of (usually) clotted blood in the septum
- Toxins — chemical damages caused by inspired substances — e.g. powdered cocaine, aerosol antihistamine medications, et cetera.
- Traumatic deformities caused by blunt trauma, penetrating trauma, and blast trauma.
- Venereal infection — e.g. syphilis
- Surgical précis
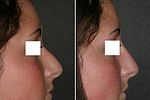
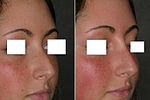
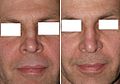
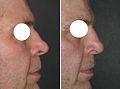
A rhinoplastic correction can be performed on a patient who is under sedation, under general anaesthesia, or under local anaesthesia; initially, a local anaesthetic mixture of lidocaine and epinephrine is injected to numb the area, and temporarily reduce vascularity, thereby limiting any bleeding. Generally, the plastic surgeon first separates the nasal skin and the soft tissues from the osseo-cartilagenous nasal framework, and then corrects (reshapes) them as required, afterwards, sutures the incisions, and then applies either an external or an internal stent, and tape, to immobilize the newly reconstructed nose, and so facilitate the healing of the surgical cuts.[1] Occasionally, the surgeon uses either an autologous cartilage graft or a bone graft, or both, in order to strengthen or to alter the nasal contour(s). The autologous grafts usually are harvested from the nasal septum, but, if it has insufficient cartilage (as can occur in a revision rhinoplasty), then either a costal cartilage graft (from the rib cage) or an auricular cartilage graft (concha from the ear) is harvested from the patient’s body. When the rhinoplasty requires a bone graft, it is harvested from either the cranium, the hips, or the rib cage; moreover, when neither type of autologous graft is available, a synthetic graft (nasal implant) is used to augment the nasal bridge.
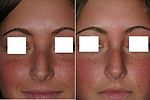
- Photographic records of the rhinoplasty
For the benefit of patient and the physician–surgeon, a photographic history of the entire rhinoplastic procedure is established; beginning at the pre-operative consultation, continuing during the surgical operation procedures, and concluding with the post-operative outcome. To record the “before-and-after” physiognomies of the nose and the face of the patient, the specific visual perspectives required are photographs of the nose viewed from the anteroposterior (front-to-back) perspective; the lateral view (profiles), the worm’s-eye view (from below), the bird’s-eye view (overhead), and three-quarter-profile views.[8]
- Sample photographic record
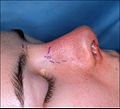
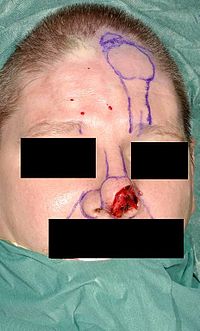
- Photograph A. — Open rhinoplasty: At rhinoplasty’s end, after the plastic surgeon has sutured (closed) the incisions, the corrected (new) nose will be dressed, taped, and splinted immobile to permit the uninterrupted healing of the surgical incisions. The purple-ink guidelines ensured the surgeon’s accurate cutting of the defect correction plan.
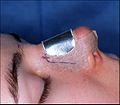
- Photograph B. — Open rhinoplasty: The new nose is prepared with paper tape in order to receive the metal nasal-splint that will immobilize it to maintain its correct shape as a new nose.
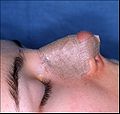
- Photograph C. — Open rhinoplasty: After the preliminary taping of the nose, a custom-made, metal nasal-splint, designed, cut, and formed by the surgeon, is emplaced to immobilize and protect the tender tissues of the new nose during convalescence.
- Photograph D. — Open rhinoplasty: The taping, emplacement of the metal splint, and dressing of the new nose complete the rhinoplasty procedure. The patient then convalesces, and the wound dressing will be removed at 1-week post-procedure.
- Sample photographic records
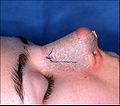
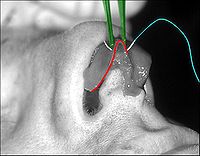
- Photograph 1. — Open rhinoplasty: The incisions are endonasal (in the nose), and thus are hidden. The skin-incision to the columella aids the plastic surgeon in precisely suturing to hide the scar — except for the columellar incision (red-dot guideline) across the nasal base. The columellar incision allows the surgeon to view the size, shape, and condition of the nasal cartilages and bones to be corrected.
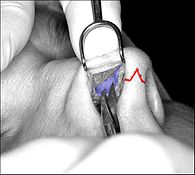
- Photograph 2. — Open rhinoplasty: The nasal interior. The scissors indicate the lower lateral cartilage (blue), which is one of the wing-shaped cartilages that conform the tip of the nose. The jagged red delineation indicates the locale of the columellar incision. Once the skin has been lifted from the bone-and-cartilage framework, the surgeon performs the nasal correction tasks.
- Photograph 3. — Open rhinoplasty: To narrow the tip of a too-wide nose, the surgeon first determines the cause of the excess nasal width. The suture being emplaced will narrow the tip of the nose. The red delineation indicates the edge of the nose-tip cartilage, which is narrowed when the surgeon tightens the folded cartilage apex. The suture (light blue) ends in the needle (white); tweezers (green) hold the nasal cartilage in place for the suturing.
- Photograph 4. — Nasal hump excision: The black delineation indicates the desired nose-reduction outcome: a straight nose. The nasal hump is bone (red) above the scalloped grey line, and cartilage (blue) below the scalloped grey line. The surgeon cuts the cartilage portion of the hump with a scalpel, and chisels the bone portion with an osteotome (bone chisel). After chiselling away the main mass of the nasal hump with an osteotome, the surgeon then sculpts, refines, and smoothens the cut nasal bones with rasps (files).
- Types of rhinoplasty
In plastic surgical praxis, the term primary rhinoplasty denotes an initial (first-time) reconstructive, functional, or aesthetic corrective procedure. The term secondary rhinoplasty denotes the revision of a failed rhinoplasty, an occurrence in 5–20 per cent of rhinoplasty operations, hence a revision rhinoplasty. The corrections usual to secondary rhinoplasty include the cosmetic reshaping of the nose because of an unaddressed nasal fracture; a defective tip of the nose, i.e. pinched (too narrow), hooked (parrot beak), or flattened (pug nose); and the restoration of clear airways. Although most revision rhinoplasty procedures are “open approach”, such a correction is more technically complicated, usually because the nasal support structures either were deformed or destroyed in the primary rhinoplasty; thus the surgeon must re-create the nasal support with cartilage grafts harvested either from the ear (auricular cartilage graft) or from the rib cage (costal cartilage graft).
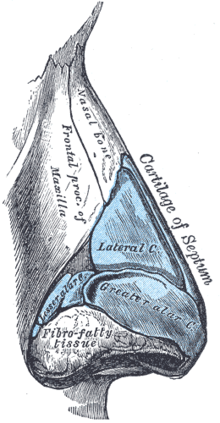 Rhinoplasty: Right lateral view of the nasal cartilages and the nasal bone.
Rhinoplasty: Right lateral view of the nasal cartilages and the nasal bone.
- Nasal reconstruction
In reconstructive rhinoplasty, the defects and deformities that the plastic surgeon encounters, and must restore to normal function, form, and appearance include broken and displaced nasal bones; disrupted and displaced nasal cartilages; a collapsed bridge of the nose; congenital defect, trauma (blunt, penetrating, blast), autoimmune disorder, cancer, intranasal drug-abuse damages, and failed primary rhinoplasty outcomes. Rhinoplasty reduces bony humps, and re-aligns the nasal bones after they are cut (dissected, resected). When cartilage is disrupted, suturing for re-suspension (structural support), or the use of cartilage grafts to camouflage a depression allow the re-establishment of the normal nasal contour of the nose for the patient. When the bridge of the nose is collapsed, rib-cartilage, ear-cartilage, or cranial-bone grafts can be used to restore its anatomic integrity, and thus the aesthetic continuity of the nose. For augmenting the nasal dorsum, autologous cartilage and bone grafts are preferred to (artificial) prostheses, because of the reduced incidence of histologic rejection and medical complications.[33]
- Surgical anatomy for nasal reconstruction
The human nose is a sensory organ that is structurally composed of three types of tissue: (i) an osseo-cartilaginous support framework (nasal skeleton), (ii) a mucous membrane lining, and (iii) an external skin. The anatomic topography of the human nose is a graceful blend of convexities, curves, and depressions, the contours of which show the underlying shape of the nasal skeleton. Hence, these anatomic characteristics permit dividing the nose into nasal subunits: (i) the midline (ii) the nose-tip, (iii) the dorsum, (iv) the soft triangles, (v) the alar lobules, and (vi) the lateral walls. Surgically, the borders of the nasal subunits are ideal locations for the scars, whereby is produced a superior aesthetic outcome, a corrected nose with corresponding skin colors and skin textures.[34][35]
- Nasal skeleton
Therefore, the successful rhinoplastic outcome depends entirely upon the respective maintenance or restoration of the anatomic integrity of the nasal skeleton, which comprises (a) the nasal bones and the ascending processes of the maxilla in the upper third; (b) the paired upper-lateral cartilages in the middle third; and (c) the lower-lateral, alar cartilages in the lower third. Hence, managing the surgical reconstruction of a damaged, defective, or deformed nose, requires that the plastic surgeon manipulate three (3) anatomic layers:
- the osseo-cartilagenous framework — The upper lateral cartilages that are tightly attached to the (rear) caudal edge of the nasal bones and the nasal septum; said attachment suspends them above the nasal cavity. The paired alar cartilages configure a tripod-shaped union that supports the lower third of the nose. The paired medial crura conform the central-leg of the tripod, which is attached to the anterior nasal spine and septum, in the midline. The lateral crura compose the second-leg and the third-leg of the tripod, and are attached to the (pear-shaped) pyriform aperture, the nasal-cavity opening at the front of the skull. The dome of the nostrils defines the apex of the alar cartilage, which supports the nasal tip, and is responsible for the light reflex of the tip.
- the nasal lining — A thin layer of vascular mucosa that adheres tightly to the deep surface of the bones and the cartilages of the nose. Said dense adherence to the nasal interior limits the mobility of the mucosa, consequently, only the smallest of mucosal defects (< 5-mm) can be sutured primarily.
- the nasal skin — A tight envelope that proceeds inferiorly from the glabella (the smooth prominence between the eyebrows), which then becomes thinner and progressively inelastic (less distensible). The skin of the mid-third of the nose covers the cartilaginous dorsum and the upper lateral cartilages and is relatively elastic, but, at the (far) distal-third of the nose, the skin adheres tightly to the alar cartilages, and is little distensible. The skin and the underlying soft tissues of the alar lobule form a semi-rigid anatomic unit that maintains the graceful curve of the alar rim, and the patency (openness) of the nostrils (anterior nares). To preserve this nasal shape and patency, the replacement of the alar lobule must include a supporting cartilage graft — despite the alar lobule not originally containing cartilage; because of its many sebaceous glands, the nasal skin usually is of a smooth (oiled) texture. Moreover, regarding scarrification, when compared to the skin of other facial areas, the skin of the nose generates fine-line scars that usually are inconspicuous, which allows the surgeon to strategically hide the surgical scars.[36]
- Principles of rhinoplastic reconstruction
The technical principles for the surgical reconstruction of a nose derive from the essential operative principles of plastic surgery: that the applied procedure and technique(s) yield the most satisfactory functional and aesthetic outcome. Hence, the rhinoplastic reconstruction of a new nasal subunit, of virtually normal appearance, can be done in a few procedural stages, using intranasal tissues to correct defects of the mucosa; cartilage battens to brace against tissue contraction and depression (topographic collapse); axial skin flaps designed from three-dimensional (3-D) templates derived from the topographic subunits of the nose; and the refinement of the resultant correction with the subcutaneous sculpting of bone, cartilage, and flesh. Nonetheless, the physician-surgeon and the rhinoplasty patient must abide the fact that the reconstructed nasal subunit is not a nose proper, but a collagen-glued collage — of forehead skin, cheek skin, mucosa, vestibular lining, nasal septum, and fragments of ear cartilage — which is perceived as a nose only because its contour, skin color, and skin texture are true to the original nose.[37]
- Restoration of the “normal nose”
In nasal reconstruction, the plastic surgeon’s ultimate goal is recreating the shadows, the contours, the skin color, and the skin texture that define the patient’s “normal nose”, as perceived at conversational distance (ca. 1.0 metre). Yet, such an aesthetic outcome suggests the application of a more complex surgical approach, which requires that the surgeon balance the patient’s required rhinoplasty, with the patient’s aesthetic ideal (body image). In the context of surgically reconstructing the patient’s physiognomy, the “normal nose” is the three-dimensional (3-D) template for replacing the missing part(s) of a nose (aesthetic nasal subunit, aesthetic nasal segment), which the plastic surgeon re-creates using firm, malleable, modelling materials — such as bone, cartilage, and flaps of skin and of tissue. In repairing a partial nasal defect (wound), such as that of the alar lobule (the dome above the nostrils), the surgeon uses the undamaged, opposite (contralateral) side of the nose as the 3-D model to fabricate the anatomic template for recreating the deformed nasal subunit, by molding the malleable template material directly upon the normal, undamaged nasal anatomy. To effect a total nasal reconstruction, the template might derive from quotidian observations of the “normal nose” and from photographs of the patient before he or she suffered the nasal damage.
The surgeon replaces missing parts with tissue of like quality and quantity; nasal lining with mucosa, cartilage with cartilage, bone with bone, and skin with skin that best match the native skin color and skin texture of the damaged nasal subunit. For such surgical repairs, skin flaps are preferable to skin grafts, because skin flaps generally are the superior remedy for matching the color and the texture of nasal skin, better resist tissue contracture, and provide better vascularisation of the nasal skeleton; thus, when there is sufficient skin to allow tissue harvesting, nasal skin is the best source of nasal skin. Furthermore, despite its notable scarring propensity, the nasal skin flap is the prime consideration for nasal reconstruction, because of its greater verisimilitude.
The most effective nasal reconstruction for repairing a defect (wound) of the nasal skin, is to re-create the entire nasal subunit; thus, the wound is enlarged to comprehend the entire nasal subunit. Technically, this surgical principle permits laying the scars in the topographic transition zone(s) between and among adjacent aesthetic subunits, which avoids juxtaposing two different types of skin in the same aesthetic subunit, where the differences of color and texture might prove too noticeable, even when reconstructing a nose with skin flaps. Nonetheless, in the final stage of nasal reconstruction — replicating the “normal nose” anatomy by subcutaneous sculpting, the surgeon does have technical allowance to revise the scars, and render them (more) inconspicuous.[38]
- Indications and techniques
- Reconstruction rhinoplasty is indicated for the correction of defects and deformities caused by
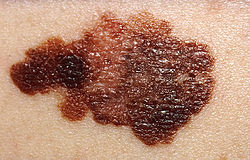
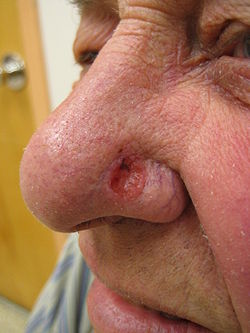
- Skin cancer. The most common cause (etiology) for a nasal reconstruction is skin cancer, especially the lesions to the nose of melanoma and basal-cell carcinoma. This oncologic epidemiology occurs more readily among the aged and people who reside in very sunny geographic areas; although every type of skin is susceptible to skin cancer, white-skin is most epidemiologically prone to developing skin cancer. Furthermore, regarding plastic surgical scars, the age of the patient is a notable factor in the timely, post-surgical healing of a skin cancer defect (lesion); in terms of scarrification, the very elastic skin of young people has a greater regenerative propensity for producing scars that are thicker (stronger) and more noticeable. Therefore, in young patients, the strategic placement (hiding) of the rhinoplastic scars is a greater aesthetic consideration than in elder patients; whose less elastic skin produces scars that are narrower and less noticeable.
- Traumatic nasal defect. Although trauma is a less common rhinoplastic occurrence, a nasal defect or deformity caused by blunt trauma (impact), penetrating trauma (piercing), and blast trauma (blunt and penetrating) requires a surgical reconstruction that abides the conservational principles of plastic surgery, as in the corrections of cancerous lesions.
- Congenital deformities. The unique plastic properties of the bone, cartilage, and skin of patients’ afflicted with congenital defects, and associated anomalies, are considered separately.[39][40]
- Surgical techniques for nasal reconstruction
The effectiveness of a rhinoplastic reconstruction of the external nose derives from the contents of the surgeon’s armamentarium of skin-flap techniques applicable to correcting defects of the nasal skin and of the mucosal lining; some management techniques are the Bilobed flap, the Nasolabial flap, the Paramedian forehead flap, and the Septal mucosal flap.
- I. The bilobed flap
The design of the bilobed flap derives from the creation of two (2) adjacent random transposition flaps (lobes). In its original design, the leading flap is applied to cover the defect, and the second flap, is emplaced where the skin flexes more, and fills the donor-site wound (from where the first flap was harvested), which then is closed primarily, with sutures. The first flap is oriented geometrically, at 90 degrees from the long axis of the wound (defect), and the second flap is oriented 180 degrees from the axis of the wound. Although effective, the bilobed flap technique did create troublesome “dog ears” of excess flesh that required trimming and it also produced a broad skin-donor area that was difficult to confine to the nose. In 1989, J. A. Zitelli modified the bilobed flap technique by: (a) orienting the leading flap at 45 degrees from the long axis of the wound; and (b) orienting the second flap at 90 degrees from the axis of the wound. Said orientations and emplacements eliminated the excess-flesh “dog ears”, and thus required a smaller area of donor skin; resultantly, the broad-based, bilobed flap is less prone to the “trap door” and the “pin cushion” deformities common to skin-flap transposition procedure.[41]
- Surgical technique — the bilobed flap
The design of the bilobed flap co-ordinates its lobes with the long axis of the nasal defect (wound); each lobe of the flap is emplaced at a 45-degree angle to the axis. The two lobes of the bilobed flap rotate along an arc, of which all points are equidistant from the apex of the nasal defect.
- Based upon the available area of nasal skin, the surgeon selects the locale for the bilobed flap, and orients the pedicle. If the defect is in the lateral aspect of the nose, the pedicle is based medially. If the defect is at the nasal tip, or at the nasal dorsum, the pedicle is based laterally. An ideal location for the second flap is along the junction of the nasal dorsum and the lateral nasal wall.
- The nasal wound is cut and shaped into a teardrop form, by the cutting out of a Burrow’s triangle of flesh on the side of pedicle base. Cutting out the Burrow’s triangle (skin and subcutaneous fat) permits the moving the pedicle flap, to emplace it without buckling the tissues adjacent to the graft.
- Using a 20-mm calliper as a protractor — one tip at the apex of the wound — the surgeon delineates two semi-circles, an inner semi-circle, and an outer semi-circle. The outer semi-circle defines the necessary length of the two lobes of the skin flap. The inner semi-circle bisects the center of the original wound, and continues across the donor skin, establishing limit measure of the pedicle common to the two lobes of the flap. The surgeon then draws two lines from the apex of the wound; the first line drawn is at an angle of 45 degrees from the long axis of the wound, and the second line drawn is at a 90-degree angle from the axis of the wound. The two (2) lines delineate the central axes of the two lobes of the bilobed flap.
- The delineation of each of the two lobes of the flap begins and ends at the inner semi-circle, and extends to the outer semi-circle, to the point where it intersects its central axis. The width of the first lobe is approximately 2-mm narrower than the width of the wound; the width of the second lobe is approximately 2-mm narrower than the width of the first lobe.
- After the cutting from the tissue donor-site, the bilobed flap is elevated to a plane between the subcutaneous fat and the nasalis muscle. The wound is deepened, down to the nasal skeleton, to accommodate the tissue thickness of the bilobed flap. Technically, cutting the wound, enlarging it, is preferable, and safer, than trimming (thinning) the flap to fit the wound.
- Undermining the donor site for the second lobe allows closing it primarily; it also eliminates excess-skin “dog-ears” at the donor site. Moreover, if the donor site cannot be closed with sutures, or if the skin blanches (whitens) when sutured, usually because of excessively tight sutures, the tension is decreased by reducing the size (length, width, depth) of the wound with deep sutures that will allow it to heal more readily.
- II. Nasolabial flap
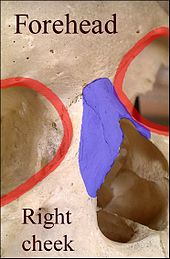
In the nineteenth century, the surgical techniques of J.F. Dieffenbach (1792–1847) popularized the nasolabial flap for nasal reconstruction, for which it remains a foundational nose surgery procedure. The nasolabial flap can be either superiorly based or inferiorly based; of which the superiorly based flap is the more practical rhinoplastic application, because it has a more versatile arc of rotation, and the donor-site scar is inconspicuous. Depending upon the how the defect lay upon the nose, the flap pedicle-base can be incorporated either solely to the nasal reconstruction, or it can be divided into a second stage procedure. The blood supply for the flap pedicle are the transverse branches of the contralateral angular artery (the facial artery terminus parallel to the nose), and by a confluence of blood vessels from the angular artery and from the supraorbital artery in the medial canthus, (the angles formed by the meeting of the upper and lower eyelids). Therefore, the incisions for harvesting the nasolabial flap do not continue superiorly beyond the medial canthal tendon. The nasolabial flap is a random flap that is emplaced with the proximal (near) portion resting upon the lateral wall of the nose, and the distal (far) portion resting upon the cheek, which contains the main angular artery, and so is perfused with retrograde arterial flow.[42]
- Surgical technique — the nasolabial flap
The pedicle of the nasolabial flap rests upon the lateral nasal wall, and is transposed a maximum of 60 degrees, in order to avoid the “bridge effect” of a flap emplaced across the nasofacial angle.
- The surgeon designs the nasolabial flap and sets its central axis at a 45-degree angle from the (long) axis of the nasal dorsum. The shape of the skin flap is cut from the wound template fabricated by the surgeon.
- An incision is made to the flap (without an anaesthetic injection of epinephrine), which then is elevated and oriented, in an inferior-to-superior direction, between the subcutaneous fat and the muscle fascia.
- The cutting continues until the skin flap can be freely transposed upon the nasal defect. A Burrow’s triangle is excised from the skin between the medial border of the flap and the nasal dorsum; the triangle can be cut either before or after the elevation of the nasolabial.
- The flap then is bent back (reflected), and can be thinned (cut) under loupe magnification; however, a nasolabial flap cannot be thinned as easily as an axial skin-flap.
- After the nasolabial flap has been emplaced, the flap donor-site wound is sutured closed. For a wound of the lateral nasal wall that is less than 15 mm wide, the flap donor-site can be closed primarily, with sutures. For a wound wider than 15 mm — especially a wound that comprehends the alar lobule and the lateral wall of the nose — primary closure is not indicated, because such a wound closure imposes excessive stresses upon the skin flap, thereby risking either blanching (whitening) or distortion, or both. Such risks are avoided by advancing (moving) the skin of the cheek towards the nasofacial junction, where it is sutured to the deep tissues. Furthermore, a narrow wound, less than 1-mm wide can be allowed to heal by secondary intention (autonomous re-epithelialisation).
- III. The paramedian forehead flap
The paramedian forehead flap is the premier autologous skin graft for the reconstruction of a nose, by replacing any of the aesthetic nasal subunits, especially regarding the problems of different tissue thickness and skin color. The forehead flap is an axial skin flap based upon the supraorbital artery (an ophthalmic artery branch) and the supratrochlear artery (an ophthalmic artery terminus), which can be thinned to the subdermal plexus in order to enhance the functional and aesthetic outcome of the nose. Restricted length is a practical application limit of the paramedian forehead flap, especially when the patient has a low frontal hairline. In such a patient, a small portion of scalp skin can be included to the flap, but it does have a different skin texture and does continue growing hair; such mismatching is avoided with the transverse emplacement of the flap along the hairline; yet that portion of the skin flap is random, and so risks a greater incidence of necrosis.
The paramedian forehead flap has two disadvantages, one operational and one aesthetic: Operationally, the reconstruction of a nose with a paramedian forehead flap is a two-stage surgical procedure, which might a problem for the patient whose health (surgical suitability) includes significant, secondary medical risks. Nonetheless, the second stage of the nasal reconstruction can be performed with the patient under local anaesthesia. Aesthetically, although the flap donor-site scar heals well, it is noticeable, and thus difficult to conceal, especially in men.[36]
- Surgical technique — the paramedian forehead flap
The surgeon designs the paramedian forehead flap from a custom-fabricated three-dimensional metal foil template derived from the measures of the nasal defect to be corrected. Using an ultrasonic scanner, the flap-pedicle is centre-aligned upon the Doppler signal of the supraorbital artery. Afterwards, the distal one-half of the flap is dissected and thinned to the subdermal plexus.
- The surgeon fabricates a metal foil template derived from the dimensions of the nasal wound.
- Applying a Doppler ultrasonic scanner, the surgeon identifies the axial pedicle of the tissue-flap (composed of the supraorbital artery and the supratrochlear artery), usually at the base, next to the medial brow; the point usually is between the midline and the supraorbital notch.
- Tracing the Doppler pulse of the blood flow of the supraorbital artery as far as possible, its delineation is continued as a vertical line, until it intersects with the hairline of the patient. The line extended from the pulse of the blood flow is the central axis of the forehead flap.
- The length of the flap is determined by placing an un-folded, un-stretched 4 x 4-inch gauze upon the wound, and with it measuring from the pedicle base to the distal (farthest) point of the wound. This measure is the length of the central axis of the skin flap.
- The template is rotated 180 degrees and placed over the distal (far) portion of the axis of the skin flap; the surgeon outlines it with a surgical marker. The outline markings are continued proximally and parallel to the central axis, maintaining a 2-cm width for the proximal flap.
- Without applying an injection of anaesthetic epinephrine, the flap is incised (cut), and the distal one-half is elevated between the frontalis muscle and the subcutaneous fat.
- At approximately the mid-portion of the forehead, the surgeon deepens the plane of the dissection down to the submuscular plane. The dissection continues toward the brow and the glabella (the smooth prominence between the eyebrows) until the skin flap is sufficiently mobile to allow its relaxed transposition upon the nose.
- Under loupe magnification, the distal portion of the forehead flap is de-fatted, down to the subdermal plexus. Yet, the fat-removal should be conservative, especially if the patient is either a tobacco smoker or a diabetic, or both, because such health factors negatively affect blood circulation and tissue perfusion, and thus the timely and correct healing of the surgical scars to the nose.
- The flap is allowed to perfuse, while the donor site is sutured closed by means of the wide undermining deep to the frontalis muscle. At that time, diluted epinephrine can be injected to the forehead skin, but not to the area(s) near the pedicle of the forehead flap. Moreover, if the distal wound is wider than 25 mm, it usually is not closed by primary intention, with sutures, but is allowed to heal by secondary intention, by re-epithelialisation.
- The forehead flap is attached to the nasal wound with subcutaneous sutures and skin sutures. If the excess tension of a suture compromises the color of the skin flap, the suture can be loosened, with a skin hook, and observed for 10–15 minutes; if the skin color remains compromised (white), the suture is removed.
- Upon the complete attachment of the paramedian forehead flap to the nose, the surgical wounds are dressed only with antibiotic ointment.
- IV. Septal mucosal flap
The septal mucosal tissue flap is the indicated technique for correcting defects of the distal half of the nose, and for correcting almost every type of large defect of the mucosal lining of the nose. The septal mucosal tissue flap, which is an anteriorly based pedicle-graft supplied with blood by the septal branch of the superior labial artery. To perform such a nasal correction, the entire septal mucoperichondrium can be harvested.[43][44]
- Surgical technique — the septal mucosal flap
The surgeon cuts the anteriorly based septal mucosal tissue-flap as widely as possible, and then releases it with a low, posterior back-cut; but only as required to allow the rotation of the tissue-flap into the nasal wound.
- The surgeon measures the dimensions (length, width, depth) of the nasal wound, and then delineates them upon the nasal septum, and, if possible, incorporates an additional margin of 3–5 mm of width to the wound measurements; furthermore, the base of the mucosal tissue flap should be at least 1.5-cm wide.
- The surgeon then makes two (2) parallel incisions along the floor and the roof of the nasal septum; the incisions converge anteriorly, towards the front of the nasal spine.
- Using an elevator, the flap is dissected in a sub-mucoperichondrial plane. The (far) distal edge of the flap is cut with a right-angle Beaver blade, and then is transposed into the wound. The exposed cartilages will reepithelialise (regenerate the epithelium), provided the opposite (contralateral) side of the septal mucosa is undisturbed.
A technical variant of the septal mucosal flap technique is the Trap-door flap, which is used to reconstruct one side of the upper half of the nasal lining. It is emplaced in the contralateral nasal cavity, as a superiorly based septal mucosal flap of rectangular shape, like that of a “trap-door”. This septomucosal flap variant is a random flap with its pedicle based at the junction of the septum and the lateral nasal skeleton. The surgeon elevates the flap of septal mucosa to the roof of the nasal septum, and then traverses it into the contralateral (opposite) nasal cavity through a slit made by removing a small, narrow portion of the dorsal roof of the septum. Afterwards, the septomucosal flap is stretched across the wound in the mucosal lining of the lateral nose.[38]
- Surgical management
The following rhinoplastic techniques are applied to the surgical management of: (i) partial-thickness defects; (ii) full-thickness defects; (iii) heminasal reconstruction; and (iv) total nasal reconstruction.
- I. Partial-thickness defects
A partial-thickness defect is a wound with adequate soft-tissue coverage of the underlying nasal skeleton, yet is too large for primary intention closure, with sutures. Based upon the locale of the wound, the surgeon has two (2) options for correcting such a wound: (i) healing the wound by secondary intention (re-epithelialisation); and (ii) healing the wound with a full-thickness skin graft. Moreover, because it avoids the patched appearance of a skin-graft surgical correction, healing by secondary intention can successfully repair nasal wounds up to 10-mm in diameter; and, if the resultant scar proves aesthetically unacceptable, it can be revised later, after the wound has healed.
In the event, larger nasal wounds (defects) do successfully heal by secondary intention, but do present two disadvantages. First, the resultant scar often is a wide patch of tissue that is aesthetically inferior to the scars produced with other nasal-defect correction techniques; however, the skin of the medial canthus is an exception to such scarring. The second disadvantage to healing by secondary intention is that the contracture of the wound might distort the normal nasal anatomy, which can lead to a pronounced deformity of the alar rim area. For this reason, healing by secondary intention generally is not recommended for defects of the distal third of the nose; nonetheless, the exception is a small wound directly upon the nasal tip.
Full-thickness skin grafts are the effective wound-management technique for defects with a well-vascularized, soft-tissue bed covering the nasal skeleton. The patient’s ear is the preferred skin-graft donor site from which to harvests grafts of pre-auricular skin and grafts of post-auricular skin, usually with an additional, small amount of adipose tissue to fill the wound cavity. Yet, nasal correction with a skin graft harvested from the patient’s neck is not recommended, because that skin is low-density pilosebaceous tissue with very few follicles and sebaceous glands, thus is unlike the oily skin of the nose.
The technical advantages of nasal-defect correction with a skin graft are a brief surgery time, a simple rhinoplastic technique, and a low incidence of tissue morbidity. The most effective corrections are with a shallow wound with sufficient, supporting soft-tissue that will prevent the occurrence of a conspicuous depression. Nonetheless, two disadvantages of skin-graft correction are mismatched skin color and skin texture, which might result in a correction with a patch-work appearance; a third disadvantage is the natural histologic tendency for such skin grafts to contract, which might distort the shape of the corrected nose.
- II. Full-thickness defects
Full-thickness nasal defects are in three types: (i) wounds to the skin and to the soft tissues, featuring either exposed bone or exposed cartilage, or both; (ii) wounds extending through the nasal skeleton; and (iii) wounds traversing all three nasal layers: skin, muscle, and the osseo-cartilaginous framework. Based upon the dimensions (length, width, depth) and topographic locale of the wound and the number of missing nasal-tissue layers, the surgeon determines the rhinoplastic technique for correcting a full-thickness defect; each of the aesthetic nasal subunits is considered separately and in combination.
- (a) Medial canthus
The skin between the nasal dorsum and the medial canthal tendon is uniquely suited to healing by secondary intention; the outcomes often are superior to what is achieved with either skin grafts or skin-flaps and tissue-flaps. Because the medial canthal tendon is affixed to the facial bone, it readily resists the forces of wound contracture; moreover, the animation (movement) of the medial brow also lends resistance to the forces of wound contracture. Furthermore, the medial canthal region is aesthetically hidden by the shadows of the nasal dorsum and of the supraorbital rim, thereby obscuring any differences in the quality of the color and of the texture of the replacement skin (epithelium).
Healing by secondary intention (re-epithelialisation) occurs even when the wound extends to the nasal bone. Although the rate of healing depends upon the patient’s wound-healing capacity, nasal wounds measuring up to 10 mm in diameter usually heal in at 4-weeks post-operative. Nonetheless, one potential, but rare, complication of this nasal correction approach is the formation of a medial canthal web, which can be corrected with two (2) opposing Z-plasties, technique which relieves the disfiguring tensions exerted by the scar tissue’s contracture, its shape, and location on the nose.
- (b) Nasal dorsum and lateral nasal wall defect
The size of the nasal defect (wound) occurred, in either the dorsum or the lateral wall, or both, determines the reconstructive skin-flap technique applicable to the corresponding aesthetic nasal subunits.
- A wound of less than 10 mm in diameter can be managed either by primary intention healing (suturing) or by secondary intention healing (re-epithelialisation).
- A wound measuring 10–15 mm in diameter can be reconstructed with a single-stage modified bilobed flap, because it best matches the skin color and the skin texture of the wounded aesthetic subunit. Although not every scar can be hidden at the margins of the aesthetic nasal subunits concerned, the superior scarring ability of those nasal skin areas minimizes such an histologic disadvantage. In a patient whose basal-cell carcinoma was excised with Mohs surgery, the scar of the nasal reconstruction (an 11-mm full-thickness, laterally based, bilobed-flap applied down to the bone and the cartilage), was hidden by aligning the axis of the second lobe to and emplacing the skin graft at the junction of the nasal dorsum and the lateral wall of the nose.
- A wound greater than 15 mm in diameter can be corrected with a paramedian forehead flap, which will reconstruct either the entire nasal dorsum or the lateral wall of the nose, as required. The surgical management of such wounds (< 15-mm dia.) usually requires enlarging the wound as necessary, in order for the skin graft to comprehend the entire aesthetic subunit being corrected. Moreover, if the wound comprehends the dorsum and the lateral wall of the nose, then a cheek-advancement skin flap is the applicable correction for replacing the lateral nasal skin up to its junction with the dorsum; afterwards, a paramedian forehead flap is applied to resurface the nasal dorsum.
- A wound in the lateral nasal wall that is greater than 15-mm in diameter can also be corrected with a superiorly based, nasolabial-flap, which is especially suited for correcting distal defects that lay among the convexities of the nasal tip and the alar lobule. The nasolabial flap can correct defects that comprehend the distal two-thirds of the nose, if there is a supply of skin sufficient for constructing the base of the flap pedicle; and the donor sites cannot be closed primarily. Yet, bulkiness is the principal disadvantage of the nasolabial flap — except in elderly patients with atrophic cheek skin; nonetheless, it is technically effective for patients unsuitable for a two-stage rhinoplasty with a paramedian forehead flap.
- Nasal defects involving either the bone or the cartilage of the lateral nose are best managed with free grafts of flat septal bone and of cartilage. Small defects of the nasal dorsum can be covered with cartilage grafts harvested from either the septum or the concha of the ear. The correction of large-area defects of the nasal dorsum requires the stable support of a bone graft affixed either with a lag screw or with a low-profile plate. A costal graft (from the rib cage) is ideal for such a repair, because it can be harvested with an attached extension of cartilage that can be sculpted to blend into the nasal tip; other potential donor sites for nasal dorsum reconstruction materials are the outer table of the skull, the iliac crest, and the inner table of the ilium proper.
- To correct a defect of the nasal lining of the upper two-thirds of the nose, the wound dimensions (length, width, depth) determine the technique. A nasal-lining defect of less than 5-mm in diameter can be closed primarily, with sutures. A nasal-lining defect 5–15 mm in diameter can be closed with a random transposition flap harvested from a nasal area that remains protected, either by the nasal bones or by the upper lateral cartilages; and the flap donor-site can be healed by secondary intention, re-epithelialisation. For a mucosa defect greater than 15-mm in diameter, the indicated correction is a superiorly based “trap door” septal mucosal flap, grafted to the roof of the nasal septum.
- (c) Nasal tip defect
The width of the human nasal-tip ranges 20–30 mm; the average width of the nasal tip, measured between the two alar lobules, is approximately 25 mm.
- A nasal skin defect of less than 15 mm in diameter can be managed with a bilobed flap; the surgeon trims the edges of the wound (defect) to match its dimensions (length, width, depth) to the natural curve at the border of the nasal tip. If the wound is eccentric, the skin-flap is positioned so that the lateral base of the graft occupies the largest portion of the wound’s surface.
- If the nasal-tip wound is greater than 15 mm in diameter, the surgeon enlarges it to comprehend the entire aesthetic subunit affected by the defect, and the reconstruction of the nasal subunit done with a forehead flap. If the nasal-tip defect also involves the nasal dorsum, a forehead flap is indicated for reconstructing the entire nasal-tip and dorsum.
- If an alar cartilage is missing, either partially or entirely, it is reconstructed with cartilage grafts. The defect of an alar dome, which retains adequate anatomic support-tripod configuration, can be corrected with an onlay graft harvested either from the nasal septum or from the conchal cartilage of an ear. The surgeon forms the cartilage graft into the shape of a shield — its widest margins become the replacement alar domes. Typically, the shield cartilage graft is stacked in two layers, in order to transmit the desired light reflex characteristic of the nasal tip.
- Defects of the lateral crura can be corrected with a flat strut of formed cartilage, but, if the support of the medial crura is absent, then a columella strut must be inserted, and attached at the level of the anterior nasal spine. If a strut of nasal-septum cartilage proves too weak, then a rib cartilage strut can be applied to provide the adequate nasal support; afterwards, the strut is covered with onlay grafts.
- Absent alar cartilages can be replaced using all of the conchal cartilage from both ears; two strips, each 10 mm wide, are harvested from the antihelical fold, and then are applied as replacement alar wings. The surgeon attaches them to the anterior nasal spine, and to each side of the (pear-shaped) pyriform aperture; the remainder of the harvested conchal cartilage is applied as onlay grafts to augment the nasal tip.
- A nasal-tip lining defect is unusual, because of its midline location; yet, the reconstruction is with an anteriorly based septal mucosal flap that is rotated into place to provide adequate coverage and correction of the nasal lining defect.
- Verticle lobule division (VLD) is a common technique for nasal tip refinement, which involves the medial crural angle and the lateral crural angle. [45]
- (d) Alar lobule defect
The appropriate surgical management of an alar lobule defect depends upon the dimensions (length, width, depth) of the wound. Anatomically, the nasal skin and the underlying soft tissues of the alar lobule form a semi-rigid aesthetic subunit that forms the graceful curve of the alar rim, and provides unobstructed airflow through the nostrils, the anterior nares.
- When most of the alar lobule tissue is missing, the nose collapses; the correction is with an ear concha cartilage-graft harvested from the antihelix, a donor site where the cartilage is most rigidly curved, thus is ideal for replacing an alar lobule.
- Nasal skin defects can be corrected with a medially based bilobed flap, which is emplaced to provide adequate skin coverage for wounds limited to the alar lobule. If the entire lobule is missing, it might be necessary to leave the second-lobe donor-site wound partially open; it will close at 2–4 weeks post-operative; afterwards, the scar can be revised. Nonetheless, the alternative surgical correction is a two-stage, superiorly based, nasolabial flap.
- If the alar lobule defect also comprehends the lateral wall of the nose, the defect can be closed either with a superiorly based nasolabial-flap or with a forehead flap. If the cheek skin is thin and atrophic, a nasolabial flap is the recommended reconstruction; otherwise, a forehead flap is recommended, because the thickness of forehead skin is a superior match for nasal skin and tissue. Mucosal lining defects of the alar lobule can be resurfaced with a bipedicled mucosal advancement-flap harvested from inside the lateral wall of the nose. Likewise, larger defects of the mucosa do require correction with an anteriorly based septal mucosal flap.
- III. Heminasal and total nasal reconstruction
The reconstruction rhinoplasty of an extensive heminasal defect or of a total nasal defect is an extension of the plastic surgical principles applied to resolving the loss of a regional aesthetic subunit. The skin layers are replaced with a paramedian forehead flap, but, if forehead skin is unavailable, the alternative corrections include the Washio retroauricular-temporal flap and the Tagliacozzi flap. The nasal skeleton is replaced with a rib-graft nasal dorsum and lateral nasal wall; septal cartilage grafts and conchal cartilage grafts are applied to correct defects of the nasal tip and of the alar lobules.[46][47]
The nasal lining of the distal two-thirds of the nose can be covered with anteriorly based septal mucosal flaps; however, if bilateral septal-flaps are used, the septal cartilage does become devascularized, possibly from iatrogenic septal perforation. Furthermore, if the nasal defect is beyond the wound-correction scope of a septal mucosal flap, the alternative techniques are either an inferiorly based pericranial-flap (harvested from the frontal bone) or a free flap of temporoparietal fascia (harvested from the head), either of which can be lined with free grafts of mucosa to achieve the nasal reconstruction.
- Corrections of defect and deformity
- Cancer — The excision of cancerous nasal skin can cause the loss of skin and internal support cartilage; such resections (surgical removal) usually are via the Mohs’ chemosurgical technique. After removing the cancerous tissues, the reconstructive rhinoplasty will provide skin coverage using either skin grafts or pedicle flaps, (see Nasal Reconstruction, Paramedian Forehead Flap). If the resection of the cancerous skin leads to losing the nose tip, cartilage grafts can be used for support, and to prevent long-term distortion consequent to the force of the contracture of scar tissue.
- Congenital deformity — The correction of vascular malformations and cleft lip and palate abnormalities. In vascular malformations, the progression of the disease distorts the skin and the underlying structure of the nose. Cleft lip and cleft palate defects usually distort the size, position, and orientation of the nasal-tip cartilages. Reconstruction of vascular malformations can involve laser treatment of the skin, and surgical excision of the deformed tissues. When the underlying cartilage support structure is disturbed, cartilage grafts and suturing of the native nasal cartilages can help improve nasal aesthetics by re-orienting the nasal tip cartilages; and cartilage-graft refinements to the nose tip are performed as required.[48]
- Obstructed airways — The restoration of normal breathing by correcting nasal obstruction caused by a cosmetic rhinoplasty wherein nasal cartilages were over-aggressively trimmed, and the nose appears pinched, which compromises nasal potency (airflow), especially when the patient attempts deep inspiration. These grafting techniques restore normal breathing by increasing the size of the nose tip with baton grafts (internal cartilage), and spreader grafts to widen the nasal middle vault. Furthermore, to improve breathing a septoplasty can be performed concurrent to the reconstructive surgery; likewise, if there is turbinate hypertrophy, an inferior turbinectomy can be performed.[49]
- Perforated septum — The reconstruction of a saddle nose caused by a (collapsed) perforated septum, or by autoimmune problems such as Wegener’s Granulomatosis, Sarcoidosis, Churg-Strauss Syndrome, Relapsing Polychondritis, by intranasal drug use, and by excessive nasal aerosol use. The saddle nose deformity resulting from lost dorsum support is reconstructed using autologous bone grafts and rib cartilage grafts.[50]
- Rhinophyma — The correction of late-stage Rosacea, wherein the nasal skin is infected with acne rosacea that reddens, thickens, and enlarges the nose tip; an exemplar case is the American actor W.C. Fields. Although antibiotic acne treatments (e.g. Acutane) can halt the progression of Rosacea, the thickened skin and the fleshy obscuring of the nasal tip can only be corrected with rhinoplasty. Laser excision of abnormally thickened skin is the best rhinoplastic treatment for Rhinophyma; the CO2 laser and the infrared Erbium: YAG laser are the most effective treatments.[51]
- Wide nose — To narrow a too-wide nose, the plastic surgeon cuts, contours, and rearranges the craniofacial bones to achieve the desired functional and aesthetic outcome of a narrower, straighter nose. To leave no visible, surgical scars upon the new nose, the surgeon effects the osteotome (bone chisel) incisions to the nasal bones beneath the facial skin.
Illustration 1: The surgeon cuts the excessively wide bones of the upper nasal dorsum (violet) with an osteotome (bone chisel), then detaches, corrects, and relocates them inwards, to a position, between the ocular orbits (red), that narrows the width of the nasal dorsum.
Illustration 2: The surgeon chisels two cuts (incisions) to the nasal bones, each incision begins at the nasal cavity. The first incision begins at the yellow dot and extends upwards, along the green arrow, until meeting the zig-zag line (red). The second incision begins at the blue dot and extends upwards, along the black arrow, until meeting the zig-zag line (red). Once cut and loosened from the face, the nasal bone pieces are corrected, then pushed inwards and re-set, thus narrowing the nose. Rhinoplasty: At 3-days post-operative, the patient wearing his nasal splint after a dorsal bone reduction, re-setting, and nasal-tip refinement. The panda eyes (orbital discoloration) is consequent to trauma and ocular blood vessel disruption.
Rhinoplasty: At 3-days post-operative, the patient wearing his nasal splint after a dorsal bone reduction, re-setting, and nasal-tip refinement. The panda eyes (orbital discoloration) is consequent to trauma and ocular blood vessel disruption.
- Post–surgical recovery
- Convalescence
The rhinoplasty patient returns home after surgery, to rest, and allow the nasal cartilage and bone tissues to heal the effects of having been forcefully cut. Assisted with prescribed medications — antibiotics, analgesics, steroids — to alleviate pain and aid wound healing, the patient convalesces for about 1-week, and can go outdoors. Post-operatively, external sutures are removed at 4–5 days; the external cast is removed at 1-week; the stents are removed within 4–14 days; and the “panda eyes” periorbital bruising heal at 2-weeks. Throughout the first year post-operative, in the course of the rhinoplastic wounds healing, the tissues will shift moderately as they settle into being a new nose.[52]
- Surgical risks
Rhinoplasty is safe, yet complications can arise; post-operative bleeding is uncommon, but usually resolves without treatment. Infection is rare, but, when it does occur, it might progress to become an abscess requiring the surgical drainage of the pus, whilst the patient is under general anaesthesia. Adhesions, scars that obstruct the airways can form a bridge across the nasal cavity, from the septum to the turbinates, and require surgical removal. If too much of the osseo-cartilaginous framework is removed, the consequent weakening can cause the external nasal skin to become shapeless, resulting in a “polly beak” deformity, resembling the beak of a parrot. Likewise, if the septum is unsupported, the bridge of the nose can sink, resulting in a “saddle nose” deformity. The tip of the nose can be over-rotated, causing the nostrils to be too visible, resulting in a porcine nose. If the cartilages of the nose tip are over-resected, it can cause a pinched-tip nose. If the columella is incorrectly cut, variable-degree numbness might result, which requires a months-long resolution. Furthermore, in the course of the rhinoplasty, the surgeon might accidentally perforate the septum (septal perforation), which later can cause chronic nose bleeding, crusting of nasal fluids, difficult breathing, and whistling breathing.
Non-surgical rhinoplasty
- The visage
Because it is the anchor-feature at the center of the face, an aesthetically proportionate nose balances the physiognomic features of a person to produce a handsome face in a man, and a beautiful face in a woman. Therefore, the rhinoplastic correction (surgical or non-surgical) of any defect or deformity (functional and aesthetic) is important, lest an aesthetically disproportionate nose distract the beholder’s attention from the other features of the face, such as the eyes, the lips, the cheekbones, and the jaw line.
- Non-surgical précis
A non-surgical rhinoplastic correction is performed upon a patient under local anaesthesia; the plastic surgeon uses a syringe and a hypodermic needle (e.g. 27-G, 25-mm) to inject and emplace the soft-tissue filler under the nasal skin, most commonly in the deep subcutaneous tissues, and, occasionally, immediately above the (periosteum), in order to correct the nasal defect or deformity, or to achieve the desired modification. Filler-injection technique makes feasible corrections such as the augmentation of a flat nasal bridge (depressed dorsum); the added projection of the nasal tip; the camouflage diminution of a nasal hump; the filling of a nasal sidewall depression; the enhancement of a retracted anterior nasal spine; the enhancement of a retracted maxilla lateral to the pyriform (pear-shaped) aperture, to displace the anterior plane; the elevation of a saddle nose deformity caused by a failed primary rhinoplasty; and traumatic injury. The technical procedures for injecting and emplacing the soft-tissue filler require approximately 1-hour to perform in the surgeon’s consultation room; thus, upon completion of the filler-injection operation, the patient convalesces at home, and resumes his or her normal life activities.
- Indications and technique
Non-surgical correction is indicated for the rhinoplasty patient who presents an innate, treatment-suitable aesthetic defect, or one occurred after a surgical rhinoplasty (either primary or secondary). Nonetheless, the functional and corrective limitations of the soft-tissue-filler medium indicate a surgical correction for the patient whose nasal defect requires extensive anatomic correction that exceeds the (aesthetic) technical scope of filler-injection procedure — which does not increase, nor does it decrease the size of the nose, and does not correct functional defects.
The corrective and technical efficacy of non-surgical rhinoplasty — achieved with syringe-and-hypodermic-needle injections of fillers to the soft tissues of the nose — was confirmed with the Australian study Rhinoplasty Using Injectable Polyacrylamide Gel — A Patient Study (2005), by Dr. Andrew Tuan-anh Le, which reported the pilot study assessment of injection techniques for emplacing a non-biodegradable colloid-filler to the soft tissues of the nose for the correction of a nasal dorsum defect; and ascertained the medical suitability of non-surgical rhinoplasty as a treatment alternative to surgical rhinoplasty.[22][23]
- Non-surgical technique
An aesthetically proportionate and functional nose for the patient was the satisfactory corrective outcome required of the assessed filler-injection techniques. In the 12-month pilot study, the nasal dorsum defects of 89 patients (84 women, 5 men, 32 yrs. avg. age) were non-surgically corrected with filler-injection treatments of polyacrylamide gel (PAAG), a non-biodegradable, hydrophilic filler for soft tissues (commercially: Aquamid). As a soft-tissue filler, PAAG is a chemically dynamic, hydrophilic colloid that continually exchanges water molecules with the surrounding nasal tissues, thus minimizing the risk of histologic rejection.
- Pre-operatively
- local anaesthesia of the pertinent aesthetic nasal subunit was via direct infiltration with lidocaine and adrenaline.
- Aseptic operating technique was achieved with a Betadine preparation complemented with alcohol swabs and hydrogen peroxide at 3.0 per cent concentration.
- Operatively
- The nasal-correction work area was the anatomic triangle delineated from the glabella (at the apex), the nasolabial folds (at the base), and the external nose proper (at the center).
- The operating field for applying the injections of the PAAG filler (polyacrylamide hydrogel) was the midline strip (1–2 mm wide) along the length of the nasal dorsum.
- For the initial injection of PAAG hydrogel filler, the syringe was perpendicular to the nasal skin surface. On penetrating the nasal skin, the surgeon advanced the hypodermic needle until it reached the nasal bone, and then penetrated under the periosteum membrane of connective tissue.
- The hydrogel filler was injected with a 27-gauge, 25-mm hypodermic needle, at bevel up, that was poised at a 30º–45° angle to the nasal area being corrected.
- The surgeon gently injected the hydrogel filler to the nasal defect, and emplaced it by simultaneously and slowly withdrawing the needle from the nose, thus dispersing the filler to the pertinent soft tissues.
- The nasal defect correction was realized with a thin wall of PAAG filler emplaced between the roof (the superficial tissues of skin- and fat-layers) and the base (the deep tissues of bone and cartilage) of the nasal dorsum.
- The corrective filler-injections of polyacrylamide hydrogel were repeated until achieving the desired degree of nasal dorsum height and shape.
- Post-operatively
- Because of the immediate, post-operative nasal swelling, consequent to the mechanical pressures of the filler-injection procedure, the height of the (new) corrected nasal bridge is approximately 20–30 per cent greater than the true height of the correction; resolution of the post-operative nasal swelling was aided with 4-times-daily applications of chloramphenicol ointment.
- At 12-months post-operative, all short-term side effects occurred had been minor and transient; the follow-up examinations of the 89-patient cohort of the pilot study indicated their satisfaction with the outcomes of their non-surgical rhinoplasty — an aesthetically proportionate nose of natural form, look, and feel.[22]
- Photographic non-surgical records
See also
- Nasal Reconstruction, Paramedian Forehead Flap
References
- ^ a b {http://www.lidlift.com/glossary/define/open-rhinoplasty.html Open Rhinoplasty Definition}
- ^ a b Muley G. Sushruta: Great Scientists of ancient India. URL: http://www.vigyanprasar.gov.in/dream/july2000.article.htm.Accessed on 07/07/2007 (s)
- ^ Chernow BA, Vallasi GA, eds. (1993) The Columbia Encyclopedia, Fifth Edition. Columbia University Press. pp. 488–489
- ^ Rinzler CA. (2009) The Encyclopedia of Cosmetic and Plastic Surgery. New York City, New York:Facts on File. p. 151
- ^ Rana RE, Arora BS. History of Plastic Surgery in India. Journal of Postgraduate Medicine. 2002;48:76–78
- ^ "Ye Olde Nose Job: The 16th Century Diagrams that Detail the World's Earliest Plastic Surgery". Daily Mail (London). 2010-12-28. http://www.dailymail.co.uk/femail/article-1341929/The-16th-century-diagrams-worlds-nose-job.html?ITO=1490.
- ^ Saraf S. Sushruta: Rhinoplasty in 600 B.C. The Internet Journal of Plastic Surgery. 2007 Volume 3, Number 2. URL: http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijps/vol3n2/rhino.xml.
- ^ a b c d e f Arneja JS. (2009) Basic Open Rhinoplasty. emedicine.medscape.com.
- ^ Rinzler CA. (2009) The Encyclopedia of Cosmetic and Plastic Surgery New York City, New York:Facts on File. p. 151
- ^ Goldwyn RM. Johann Friedrich Dieffenbach (1794–1857). Plastic and Reconstructive Surgery. July 1968, Volume 42, Issue 1, pp. 19–28
- ^ Roe JO. The Deformity Termed “Pug Nose” and its Correction, by a Simple Operation. 31. New York: The Medical Record; 1887:621.
- ^ Rinzler CA. (2009) The Encyclopedia of Cosmetic and Plastic Surgery. New York City, New York:Facts on File. pp. 151–152
- ^ Rethi A. Operation to Shorten an Excessively Long Nose. Revue de Chirurgie Plastique. 1934;2:85
- ^ Goodman WS, Charles DA. Technique of External Rhinoplasty. Journal of Otolaryngology. February 1978;7(1):13–17.
- ^ Gunter JP. The Merit of the Open Approach in Rhinoplasty. Plastic and Reconstructive Surgery. March 1997;99(3):863–867.
- ^ Goodman WS (1973). "External Approach to Rhinoplasty". Canadian Journal of Otolaryngology 2 (3): 207–10. PMID 4791580.
- ^ Anderson JR, Johnson CM, Adamson P (1982). "Open Rhinoplasty: An Assessment". Otolaryngology—Head and Neck Surgery 90 (2): 272–4. PMID 6810277.
- ^ Gunter JP, Rohrich RJ (August 1987). "External Approach for Secondary Rhinoplasty". Plastic and Reconstructive Surgery 80 (2): 161–174. doi:10.1097/00006534-198708000-00001. PMID 3602167.
- ^ Rinzler CA. (2009) The Encyclopedia of Cosmetic and Plastic Surgery. New York City, New York:Facts on File. pp. 164–65
- ^ Wilke TF. Late Development of Granulomas after Liquid Silicone Injections. Plastic and Reconstructive Surgery. 60(2):179. 1977
- ^ Orentreich DS. Liquid Injectable Silicone — Techniques for Soft tissue Augmentation. Clinics in Plastic Surgery. 27(4):595, 2000
- ^ a b c Le, Andrew Tuan-anh. Rhinoplasty Using Injectable Polyacrylamide Gel — A Patient Study. Australasian Journal of Cosmetic Surgery. 2005 Volume 1, No. 1
- ^ a b BHC — Non-surgical Rhinoplasty Sydney — Beauty and Health Care Sydney Injectable Rhinoplasty.
- ^ Hengerer AS, Oas RE. Congenital Anomalies of the Nose: Their Embryology, Diagnosis, and Management (SIPAC). Alexandria, Virginia: American Academy of Otolaryngology; 1987.
- ^ Chang EW. Nose Anatomy. emedicine.medscape.com.
- ^ Fattahi T. An Overview of Facial Aesthetic Units. Journal of Oral and Maxillofacial Surgery. Volume 61, Issue 10, pp. 1207–1221. URL: http://www.med-ars.it/galleries/aesthetic_units_nose.htm.
- ^ Heidari Z, Mahmoudzadeh-Sagheb H, Khammar T, Khammar M. Anthropometric Measurements of the External Nose in 18–25-year-old Sistani and Baluch Aborigine Women in the southeast of Iran. Folia Morphologiica (Warszawa). May 2009;68(2):88–92.
- ^ Burget GC, Menick FJ. The Subunit Principle in Nasal Reconstruction. Plastic and Reconstructive Surgery. August 1985;76(2):239–247.
- ^ Bailey BJ, Johnson JT, Newlands SD. Head and Neck Surgery — Otolaryngology. Lippincott Williams & Wilkins. 2006 p. 2,490
- ^ Heinberg CE, Kern EB. The Cottle sign: An Aid in the Physical Diagnosis of Nasal Airflow Disturbances. Rhinology. 1973;11:89–94.
- ^ Vartanian AJ. Basic Closed Rhinoplasty. Emedicine.medscape.com.
- ^ Hopping SB, White JB. Ethnic Rhinoplasty: a Universal Preoperative Classification System for the Nasal Tip. The American Journal of Cosmetic Surgery 2009;26(1):35–39.
- ^ Stanley RB, Schwartz MS (October 1989). "Immediate reconstruction of contaminated central craniofacial injuries with free autogenous grafts". The Laryngoscope 99 (10 Pt 1): 1011–5. doi:10.1288/00005537-198210000-00007. PMID 2796548.
- ^ Millard DR Jr. Aesthetic Reconstructive Rhinoplasty. Clinics in Plastic Surgery. April 1981;8(2):169–175.
- ^ Burget GC. Aesthetic Restoration of the Nose. Clinics in Plastic Surgery. July 1985;12(3):463–480.
- ^ a b Fata J. (2008) Nasal Reconstruction: Principles and Techniques, emedicine.medscape.com
- ^ Burget GC. Aesthetic Restoration of the Nose. Clinics in Plastic Surgery. July 1985;12(3):463–80.
- ^ a b Fata J. (2008) Nasal Reconstruction: Principles and Techniques. emedicine.medscape.com.
- ^ Fata J. (2008) Nasal Reconstruction: Principles and Techniques, emedicine.medscape.com.
- ^ Vartanian AJ. Saddle Nose Rhinoplasty.|URL: http://emedicine.medscape.com/article/840910-overview#showall
- ^ Zitelli JA. The Bilobed flap for Nasal Reconstruction. Archives of Dermatology. July 1989;125(7):957–959.
- ^ Dieffenbach JF. Die Nasenbehändlung in Operative Chirurgie. In: Brockhaus FA, ed. Leipzig. 1845.
- ^ Millard DR Jr. Hemirhinoplasty. Plastic and Reconstructive Surgery. November 1967;40(5):440–445.
- ^ Burget GC, Menick FJ. Nasal Support and Lining: the Marriage of Beauty and Blood Supply. Plastic and Reconstructive Surgery. August 1989;84(2):189–202.
- ^ Funk, Etai. Nasal Tip Dynamics. 2008, p. 1. funkfacialplastics.com
- ^ Washio H. Retroauricular-temporal Flap. Plastic and Reconstructive Surgery. February 1969;43(2):162–166.
- ^ Tagliacozzi G, Gaspare B. editor, Venice. De Curtorum Chirurgia per Insitionem (1597).
- ^ Wang TD, Madorsky SJ (1999). "Secondary rhinoplasty in nasal deformity associated with the unilateral cleft lip". Archives of Facial Plastic Surgery 1 (1): 40–5. doi:10.1001/archfaci.1.1.40. PMID 10937075.
- ^ Khosh MM, Jen A, Honrado C, Pearlman SJ (2004). "Nasal valve reconstruction: experience in 53 consecutive patients". Archives of Facial Plastic Surgery 6 (3): 167–71. doi:10.1001/archfaci.6.3.167. PMID 15148124.
- ^ Tardy ME, Schwartz M, Parras G (1989). "Saddle nose deformity: autogenous graft repair". Facial Plastic Surgery 6 (2): 121–34. doi:10.1055/s-2008-1064719. PMID 2487867.
- ^ Rohrich RJ, Griffin JR, Adams WP (September 2002). "Rhinophyma: review and update". Plastic and Reconstructive Surgery 110 (3): 860–69; quiz 870. doi:10.1097/00006534-200209010-00023. PMID 12172152.
- ^ Rhinoplasty Surgery Recovery Plastic Surgery Guide
- Talmant JC, Talmant JC, Lumineau JP (September 2007). "[Nasal sequels of unilateral clefts: analysis and management]" (in French). Revue De Stomatologie et De Chirurgie Maxillo-faciale 108 (4): 275–88. doi:10.1016/j.stomax.2007.06.011 (inactive 2010-02-28). PMID 17688895.
- Swanepoel PF, Fysh R (2007). "Laminated dorsal beam graft to eliminate postoperative twisting complications". Archives of Facial Plastic Surgery 9 (4): 285–9. doi:10.1001/archfaci.9.4.285. PMID 17638765.
- Inanli S, Sari M, Baylancicek S (2007). "The use of expanded polytetrafluoroethylene (Gore-Tex) in rhinoplasty". Aesthetic Plastic Surgery 31 (4): 345–8. doi:10.1007/s00266-007-0037-z. PMID 17549553.
- Arslan E, Aksoy A (June 2007). "Upper lateral cartilage-sparing component dorsal hump reduction in primary rhinoplasty". The Laryngoscope 117 (6): 990–6. doi:10.1097/MLG.0b013e31805366ed. PMID 17545863.
- Cochran CS, Ducic Y, DeFatta RJ (May 2007). "Restorative rhinoplasty in the aging patient". The Laryngoscope 117 (5): 803–7. doi:10.1097/01.mlg.0000248240.72296.b9. PMID 17473672.
- Hellings PW, Nolst Trenité GJ (June 2007). "Long-term patient satisfaction after revision rhinoplasty". The Laryngoscope 117 (6): 985–9. doi:10.1097/MLG.0b013e31804f8152. PMID 17460577.
- Thomson C, Mendelsohn M (April 2007). "Reducing the incidence of revision rhinoplasty". The Journal of Otolaryngology 36 (2): 130–4. doi:10.2310/7070.2007.0012. PMID 17459286.
- Reilly MJ, Davison SP (2007). "Open vs closed approach to the nasal pyramid for fracture reduction". Archives of Facial Plastic Surgery 9 (2): 82–6. doi:10.1001/archfaci.9.2.82. PMID 17372060.
- Patrocínio LG, Patrocínio JA (October 2007). "Open rhinoplasty for African-American noses". The British Journal of Oral & Maxillofacial Surgery 45 (7): 561–6. doi:10.1016/j.bjoms.2007.01.011. PMID 17350737.
- Gurney TA, Kim DW (February 2007). "Applications of porcine dermal collagen (ENDURAGen) in facial plastic surgery". Facial Plastic Surgery Clinics of North America 15 (1): 113–21, viii. doi:10.1016/j.fsc.2006.10.007. PMID 17317562.
- Gruber R, Chang TN, Kahn D, Sullivan P (March 2007). "Broad nasal bone reduction: an algorithm for osteotomies". Plastic and Reconstructive Surgery 119 (3): 1044–53. doi:10.1097/01.prs.0000252504.65746.18. PMID 17312512.
- Gubisch W (November 2006). "Twenty-five years experience with extracorporeal septoplasty". Facial Plastic Surgery 22 (4): 230–9. doi:10.1055/s-2006-954841. PMID 17131265.
- Thornton MA, Mendelsohn M (November 2006). "Total skeletal reconstruction of the nasal dorsum". Archives of Otolaryngology--Head & Neck Surgery 132 (11): 1183–8. doi:10.1001/archotol.132.11.1183. PMID 17116812.
- Pedroza F, Anjos GC, Patrocinio LG, Barreto JM, Cortes J, Quessep SH (2006). "Seagull wing graft: a technique for the replacement of lower lateral cartilages". Archives of Facial Plastic Surgery 8 (6): 396–403. doi:10.1001/archfaci.8.6.396. PMID 17116787.
- Romo T, Kwak ES, Sclafani AP (2006). "Revision rhinoplasty using porous high-density polyethylene implants to re-establish ethnic identity". Aesthetic Plastic Surgery 30 (6): 679–84; discussion 685. doi:10.1007/s00266-006-0049-0. PMID 17093875.
- Romo T, Kwak ES (November 2006). "Difficult revision case: Overaggressive resection". Facial Plastic Surgery Clinics of North America 14 (4): 411–5, viii. doi:10.1016/j.fsc.2006.06.009. PMID 17088190.
- Boccieri A, Macro C (November 2006). "Difficult revision case: Two previous septo-rhinoplasties". Facial Plastic Surgery Clinics of North America 14 (4): 407–9, viii. doi:10.1016/j.fsc.2006.06.013. PMID 17088189.
- Toriumi DM (November 2006). "Difficult revision case: Foreshortened nose and severe alar retraction, two prior rhinoplasty surgeries". Facial Plastic Surgery Clinics of North America 14 (4): 401–6, viii. doi:10.1016/j.fsc.2006.06.012. PMID 17088188.
External links
- Rhinoplasty Surgery - Guide University of Maryland plastic surgery guides series
Respiratory system surgeries and other procedures (ICD-9-CM V3 21-22, 30-34, ICD-10-PCS 0B) Upper RT nose: Rhinoplasty · Septoplasty · Rhinectomy · Rhinomanometry
larynx: Laryngoscopy · Laryngectomy · Laryngotomy (Thyrotomy)Lower RT trachea: Cricothyrotomy · Tracheoesophageal puncture · Tracheotomy
lung: Pneumonectomy · Wedge resection · Lung transplantation · Decortication of lung · Heart-lung transplantChest wall, pleura,
mediastinum, and diaphragmMedical imaging Bronchography · CT pulmonary angiogram · High resolution CT · Spiral CT · Ventilation/perfusion scanCPRs Lung function test Cytology Respiratory therapy/
intubationCategories:- Plastic surgery
- Nose surgery
Wikimedia Foundation. 2010.