- 1,1,1-Trichloroethane
-
Not to be confused with 1,1,1-Trifluoroethane.
1,1,1-Trichloroethane 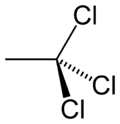
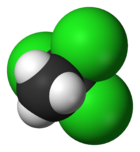 1,1,1-trichloroethaneOther namesmethyl chloroform, chlorothene, Solvent 111, Genklene
1,1,1-trichloroethaneOther namesmethyl chloroform, chlorothene, Solvent 111, GenkleneIdentifiers CAS number 71-55-6 
PubChem 6278 ChemSpider 6042 
UNII 113C650IR1 
KEGG C18246 
ChEBI CHEBI:36015 
ChEMBL CHEMBL16080 
Jmol-3D images Image 1 - ClC(Cl)(Cl)C
Properties Molecular formula C2H3Cl3 or CH3CCl3 Molar mass 133.40 g/mol Appearance Colorless liquid Density 1.32 g/cm3 Melting point -33 °C, 240 K, -27 °F
Boiling point 74 °C, 347 K, 165 °F
Solubility in water insoluble in water Hazards R-phrases R19 R20 R40 R59 R66 S-phrases S9 S16 S24 S25 S46 S59 S61 Main hazards Irritant to the upper respiratory tract. Causes severe irritation and swelling to eyes. NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent.[1] It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.
Contents
Production
1,1,1-Trichloroethane was first reported by Henri Victor Regnault in 1840. Industrially, it is usually produced in a two-step process from vinyl chloride. In the first step, vinyl chloride reacts with hydrogen chloride at 20-50 °C to produce 1,1-dichloroethane:
- CH2=CHCl + HCl → CH3CHCl2
This reaction is catalyzed by a variety of Lewis acids, mainly aluminium chloride, iron(III) chloride, or zinc chloride. The 1,1-dichloroethane is then converted to 1,1,1-trichloroethane by reaction with chlorine under ultraviolet irradiation:
- CH3CHCl2 + Cl2 → CH3CCl3 + HCl
This reaction proceeds at 80-90% yield, and the hydrogen chloride byproduct can be recycled to the first step in the process. The major side-product is the related compound 1,1,2-trichloroethane, from which the 1,1,1-trichloroethane can be separated by distillation.
A somewhat smaller amount of 1,1,1-trichloroethane is produced from the reaction of vinylidene chloride and hydrogen chloride in the presence of an iron(III) chloride catalyst:
- CH2=CCl2 + HCl → CH3CCl3
1,1,1-Trichloroethane is marketed with stabilizers since it is unstable with respect to dehydrochlorination and attacks some metals. Stabilizers comprise up to 8% of the formulation, including acid scavengers (epoxides, amines) and complexants. The Montreal Protocol targeted 1,1,1-trichloroethane as one of those compounds responsible for ozone depletion and banned its use beginning in 1996. Since then, its manufacture and use has been phased out throughout most of the world.
Uses
1,1,1-Trichloroethane is generally considered as a nonpolar solvent. Owing to its unsymmetrical structure, it is a superior solvent for organic compounds that do not dissolve well in hydrocarbons such as hexane. It is an excellent solvent for many organic materials and also one of the least toxic of the chlorinated hydrocarbons. Prior to the Montreal Protocol, it was widely used for cleaning metal parts and circuit boards, as a photoresist solvent in the electronics industry, as an aerosol propellant, as a cutting fluid additive, and as a solvent for inks, paints, adhesives and other coatings.
It was also the standard cleaner for photographic film (movie/slide/negatives, etc.). Other commonly available solvents damage emulsion, and thus are not suitable for this application. The standard replacement, Forane 141 is much less effective, and tends to leave a residue. 1,1,1-Trichloroethane was used as a thinner in Correction fluid products such as Liquid paper. Many applications for 1,1,1-trichloroethane (including film cleaning) were previously done with carbon tetrachloride, which was banned in 1970. In the laboratory, 1,1,1-trichloroethane is being been replaced by other solvents.[2]
1,1,1-Trichloroethane is used as an insecticidal fumigant.[3]
Safety
Although not as toxic as many similar compounds, inhaled or ingested 1,1,1-trichloroethane does act as a central nervous system depressant and can cause effects similar to those of intoxication, including dizziness, confusion, and in sufficiently high concentrations, unconsciousness and death.[4] Fatal poisonings have been reported.[5][6][7] and illness[8] linked to trichloroethane from intentional inhalation in the 1980s. The removal of the chemical from correction fluid commenced due to Proposition 65 declaring it hazardous and toxic[9][10]
Prolonged skin contact with the liquid can result in the removal of fats from the skin, resulting in chronic skin irritation. Studies on laboratory animals have shown that 1,1,1-trichloroethane is not retained in the body for long periods of time. However, chronic exposure has been linked to abnormalities in the liver, kidneys, and heart. Pregnant women should avoid exposure, as the compound has been linked to birth defects in laboratory animals (see teratogenesis).
References
- ^ Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, Trevor Mann “Chlorinated Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2.
- ^ Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003
- ^ Methylchloroform data sheet, alanwood.net
- ^ Toxicological Profile for 1,1,1-Trichloroethane, Agency for Toxic Substances and Disease Registry (ATSDR). 2006
- ^ King, Gregory S.; Smialek, John E.; Troutman, William G. (15 March 1985). "Sudden Death in Adolescents Resulting From the Inhalation of Typewriter Correction Fluid". JAMA : the Journal of the American Medical Association 253 (11): 1604–1606. doi:10.1001/jama.253.11.1604. PMID 3974043. Archived from the original on 2009-07-25. http://jama.ama-assn.org/cgi/content/short/253/11/1604. Retrieved 2010-01-05. "We describe four cases of sudden death in adolescents associated with recreational sniffing of typewriter correction fluid occurring during the period 1979 through mid-1984."
- ^ D'costa, DF; Gunasekera, NP (August 1990). "Fatal cerebral oedema following trichloroethane abuse". Journal of the Royal Society of Medicine 83 (8): 533–534. PMC 1292788. PMID 2231588. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1292788.
- ^ Winekab, Charles L.; Wahba, Wagdy W.; Huston, Robert; Rozin, Leon (6 June 1997). "Fatal inhalation of 1,1,1-trichloroethane". Forensic Science International 87 (2): 161–165. doi:10.1016/S0379-0738(97)00040-6. PMID 9237378. Archived from the original on 2009-07-25. http://www.fsijournal.org/article/PIIS0379073897000406/abstract. Retrieved 2010-01-05. "A 13-year-old male was found dead in the woods subsequent to 1,1,1-trichloroethane (TCE) inhalation."
- ^ Wodka, Richard M.; Jeong, Erwin W. S. (1 January 1989). "Cardiac Effects of Inhaled Typewriter Correction Fluid". Annals of Internal Medicine 110 (1): 91–92. doi:10.1059/0003-4819-110-1-91_2. PMID 2908837. Archived from the original on 2009-07-25. http://www.annals.org/cgi/content/abstract/110/1/91-a. Retrieved 2010-01-05.
- ^ Paddock, Richard C. (29 September 1989). "Gillette Agrees to Remove Toxics From Its Paper Correction Fluid". Sacramento: Los Angeles Times. Archived from the original on 22 July 2009. http://www.webcitation.org/5iTIv5LFW. Retrieved 5 January 2010.
- ^ Estrin, Norman F.; Akerson, James M. (2000). "Proposition 65". Cosmetic regulation in a competitive environment. New York, New York: Marcel Dekker. p. 138. ISBN 0824775163. http://books.google.com/?id=O8z3Nn9HzKIC&pg=PA138. Retrieved 5 January 2010. "Gillette agreed to reformulate the product so that it would not pose a risk requiring a Proposition 65 warning"
Further reading
- Doherty, R.E. 2000. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 2 - Trichloroethylene and 1,1,1-Trichloroethane, Journal of Environmental Forensics (2000) 1, 83-93.
Categories:- Greenhouse gases
- Hazardous air pollutants
- Organochlorides
- Halogenated solvents
- Excipients
Wikimedia Foundation. 2010.

