- Adinazolam
-
Adinazolam 

Systematic (IUPAC) name 1-(8-chloro-6-phenyl-4H-[1,2,4]triazolo[4,5-a]
[1,4]benzodiazepin-1-yl)-N,N-dimethylmethanamineClinical data Pregnancy cat. ? Legal status Schedule IV(US) Routes Oral Pharmacokinetic data Bioavailability ? Metabolism Hepatic Half-life < 3 hours Excretion Renal Identifiers CAS number 37115-32-5 
ATC code N05BA07 PubChem CID 37632 DrugBank APRD00724 ChemSpider 34519 
UNII KN08449444 
KEGG D02770 
ChEBI CHEBI:251412 
ChEMBL CHEMBL328250 
Chemical data Formula C19H18ClN5 Mol. mass 351.8 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Adinazolam[1] (marketed under the brand name Deracyn) is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative, and antidepressant[2] properties. Adinazolam was developed by Dr. Jackson B. Hester, who was seeking to enhance the antidepressant properties of alprazolam, which he also developed.[3]
Contents
Indications
Adinazolam is indicated as a treatment for anxiety and status epilepticus.
Pharmacology
Adinazolam produces inhibitory effects by binding to GABA receptors. This increases the effects of GABA.
Metabolism
Adinazolam was reported to have active metabolites in the August 1984 issue of The Journal of Pharmacy and Pharmacology.[4] The main metabolite is N-desmethyladinazolam.[5] The other two metabolites are alpha-hydroxyalprazolam and estazolam.[6] In the August 1986 issue of that same journal, Sethy, Francis and Day reported that proadifen inhibited the formation of N-desmethyladinazolam.[7]
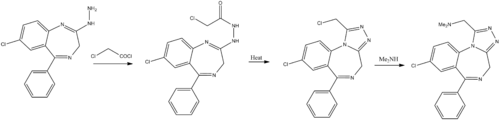
Chemistry
Hester, J. B.; Rudzik, A. D.; Von Voigtlander, P. F.; J. Med. Chem. 1980, 23, 392.
http://dx.doi.org/10.1021/jm00178a009
See also
References
- ^ FR Patent 2248050
- ^ Lahti, Robert A.; Vimala H. Sethy, Craig Barsuhn, Jackson B. Hester (November 1983). "Pharmacological profile of the antidepressant adinazolam, a triazolobenzodiazepine.". Neuropharmacology 22 (11): 1277–82. doi:10.1016/0028-3908(83)90200-9. PMID 6320036.
- ^ "Discovers Award 2004" (PDF). Special Publications. Pharmaceutical Research and Manufactureres of America. April 2004. pp. 39. Archived from the original on August 24, 2006. http://web.archive.org/web/20060824202348/http://international.phrma.org/publications/publications/admin/2004-10-12.1086.pdf. Retrieved August 18, 2006.
- ^ Sethy, Vimala H.; R. J. Collins and E. G. Daniels (August 1984). "Determination of biological activity of adinazolam and its metabolites.". Journal of Pharmacy and Pharmacology 36 (8): 546–8. doi:10.1111/j.2042-7158.1984.tb04449.x. PMID 6148400.
- ^ Peng, G. W. (August 1984). "Assay of adinazolam in plasma by liquid chromatography". Journal of Pharmaceutical Sciences 73 (8): 1173–5. doi:10.1002/jps.2600730840. PMID 6491930.
- ^ Fraser, A. D.; A. F. Isner and W. Bryan (November-December 1993). "Urinary screening for adinazolam and its major metabolites by the Emit d.a.u. and FPIA benzodiazepine assays with confirmation by HPLC". Journal of Analytical Toxicology 17 (7): 427–31. PMID 8309217.
- ^ Sethy, Vimala H.; Jonathan W. Francis and J. S. Day (August 1986). "The effect of proadifen on the metabolism of adinazolam". Journal of Pharmacy and Pharmacology 38 (8): 631–2. doi:10.1111/j.2042-7158.1986.tb03099.x. PMID 2876087.
Benzodiazepine derivatives 1,4-Benzodiazepines Bromazepam · Camazepam · Carburazepam · Chlordiazepoxide · Cinolazepam · Clonazepam · Clorazepate · Cyprazepam · Delorazepam · Demoxepam · Devazepide * · Diazepam · Doxefazepam · Elfazepam · Ethyl carfluzepate · Ethyl dirazepate · Ethyl loflazepate · Fletazepam · Fludiazepam · Flunitrazepam · Flurazepam · Flutemazepam · Flutoprazepam · Fosazepam · Gidazepam · Halazepam · Iclazepam · Ketazolam · Lorazepam · Lormetazepam · Meclonazepam · Medazepam · Menitrazepam · Metaclazepam · Motrazepam · Nimetazepam · Nitrazepam · Nitrazepate · Nordazepam · Nortetrazepam · Oxazepam · Phenazepam · Pinazepam · Pivoxazepam · Prazepam · Proflazepam · Quazepam · QH-II-66 · Reclazepam · Ro5-2904 · Ro5-4864 * · Sulazepam · Temazepam · Tetrazepam · Tifluadom * · Tolufazepam · Tuclazepam · Uldazepam
1,5-Benzodiazepines Arfendazam · Clobazam · CP-1414S · Lofendazam · Triflubazam
2,3-Benzodiazepines * Girisopam · GYKI-52466 · GYKI-52895 · Nerisopam · Talampanel · Tofisopam
Triazolobenzodiazepines Adinazolam · Alprazolam · Estazolam · Flubromazolam · Triazolam
Imidazobenzodiazepines Bretazenil · Climazolam · FG-8205 · Flumazenil · Imidazenil · L-655,708 · Loprazolam · Midazolam · PWZ-029 · Remimazolam · Ro15-4513 · Ro48-6791 · Ro48-8684 · Sarmazenil · SH-053-R-CH3-2′F
Oxazolobenzodiazepines Cloxazolam · Flutazolam · Haloxazolam · Mexazolam · Oxazolam
Thienodiazepines Bentazepam · Brotizolam · Ciclotizolam · Clotiazepam · Etizolam · Olanzapine *
Pyridodiazepines Lopirazepam · Zapizolam
Pyrazolodiazepines Pyrrolodiazepines Tetrahydroisoquinobenzodiazepines Benzodiazepine prodrugs * atypical activity profile (not GABAA receptor ligands)
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.