- Mescaline
-
Mescaline 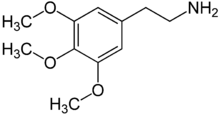
Systematic (IUPAC) name 2-(3,4,5-trimethoxyphenyl)ethanamine Clinical data AHFS/Drugs.com entry Pregnancy cat. C(US) Legal status Prohibited (S9) (AU) Schedule III (CA) ? (UK) Schedule I (US) Routes Oral, Intravenous Pharmacokinetic data Half-life 6 hours Identifiers CAS number 54-04-6 
ATC code None PubChem CID 4076 ChemSpider 3934 
UNII RHO99102VC 
KEGG C06546 
ChEBI CHEBI:28346 
ChEMBL CHEMBL26687 
Synonyms 3,4,5-trimethoxyphenethylamine Chemical data Formula C11H17NO3 Mol. mass 211.257 g/mol SMILES eMolecules & PubChem Physical data Melt. point 183 °C (361 °F)  (what is this?) (verify)
(what is this?) (verify)Mescaline or 3,4,5-trimethoxyphenethylamine is a naturally occurring psychedelic alkaloid of the phenethylamine class used mainly as an entheogen.
It occurs naturally in the peyote cactus (Lophophora williamsii),[1] and the Peruvian Torch cactus (Echinopsis peruviana), and in a number of other members of the Cactaceae plant family. It is also found in small amounts in certain members of the Fabaceae (bean) family, including Acacia berlandieri.[2] Mescaline was first isolated and identified in 1897 by the German Arthur Heffter and first synthesized in 1919 by Ernst Späth.
Contents
History and usage
Peyote has been used for over 3000 years by Native Americans in Mexico.[1] Europeans noted use of peyote in Native American religious ceremonies upon early contact, notably by the Huichols in Mexico. Other mescaline-containing cacti such as the San Pedro have a long history of use in South America, from Peru to Ecuador.
In traditional peyote preparations the top of the cactus is cut at ground level, leaving the large tap roots to grow new 'Heads'. These 'Heads' are then dried to make disk-shaped buttons. Buttons are chewed to produce the effects or soaked in water for an intoxicating drink. However, the taste of the cactus is bitter, so users will often grind it into a powder and pour it in capsules to avoid having to taste it. The usual human dosage is 200–400 milligrams of mescaline sulfate or 178–356 milligrams of mescaline hydrochloride.[3] The average 3-inch (76 mm) button contains about 25 mg mescaline.[4]
Aldous Huxley described his experience with mescaline in The Doors of Perception. Aleister Crowley reported using mescaline in his diary. The sex psychologist Havelock Ellis also tried mescaline.[5] Hunter S. Thompson recounted an extremely detailed account of his first use of mescaline in First Visit with Mescalito, appearing in his book Songs of the Doomed.
Biosynthesis of Mescaline
Mescaline can be synthesized from tyrosine or a hydroxylated phenylalanine. In Lophophora williamsii, dopamine converts into mescaline in a biosynthetic pathway involving m-O-methylation and aromatic hydroxylation.[6]
Pharmacokinetics
Tolerance builds with repeated usage, lasting for a few days. Mescaline causes cross-tolerance with some other psychedelics such as LSD/LSA, DMT, and psilocybin. THC, and muscimol most likely do not produce cross tolerance with each other or with the LSD-LSA-mescaline-psilocybin-DMT group.[7]
About half the initial dosage is excreted after 6 hours, but some studies suggest that it is not metabolized at all before excretion. Mescaline appears to not be subject to metabolism by CYP2D6[8] and between 20 and 50% of mescaline is excreted in the urine unchanged, and the rest being excreted as the carboxylic acid form of mescaline, a likely result of MAO degradation.[9] The LD50 of mescaline has been measured in various animals: 212 mg/kg i.p. (mice), 132 mg/kg i.p. (rats), and 328 mg/kg i.p. (guinea pigs).
Behavioral and non-behavioral effects
The visual distortions produced by mescaline are somewhat different from those of LSD. The subjective "open-eye visuals" are not true hallucinations as they are consistent with actual experience and manifest as intensifications and alterations of existing stimuli (objects and sounds), not the appearance of non-existent fanciful objects or actions that the user believes are real; the appearance of non-existent persons or objects is a feature of true hallucinations, and believing that the hallucinations are "real" is a defining feature of delusions, which is not a common effect of mescaline. Prominence of color is distinctive, appearing brilliant and intense. Recurring visual patterns observed during the mescaline experience include stripes, checkerboards, angular spikes, multicolored dots, and very simple fractals which turn very complex. Aldous Huxley described these self transforming amorphous shapes as like animated stained glass illuminated from light coming through the eyelids. Like LSD, mescaline induces distortions of form and kaleidoscopic experiences but which manifest more clearly with eyes closed and under low lighting conditions; however, all of these visual descriptions are purely subjective.
As with LSD, synesthesia can occur especially with the help of music.[10] An unusual but unique characteristic of mescaline use is the "geometricization" of three-dimensional objects. The object can appear flattened and distorted, similar to the presentation of a Cubist painting.[11]
Mescaline elicits a pattern of sympathetic arousal, with the peripheral nervous system being a major target for this drug.[10] Effects typically last for 12-18 hours.
Mode of action
Mescaline acts similarly to other psychedelic agents.[12] It binds to and activates the serotonin 5-HT2A receptor with a high affinity as a partial agonist.[13] How activating the 5-HT2A receptor leads to psychedelia is still unknown, but it likely somehow involves excitation of neurons in the prefrontal cortex.[14] Mescaline is also known to bind to and activate the serotonin 5-HT2C receptor.[15]
In addition to serotonin receptor activity, mescaline also stimulates the dopamine receptors.[16] Whether mescaline possesses dopamine receptor agonist properties or initiates the release of dopamine remains unclear.
Legality
In the United States, mescaline was made illegal in 1970 by the Comprehensive Drug Abuse Prevention and Control Act.[17] The drug was prohibited internationally by the 1971 Convention on Psychotropic Substances[18] and is categorized as a Schedule I hallucinogen by the CSA. Mescaline is legal only for certain religious groups (such as the Native American Church) and in scientific and medical research. In 1990, the Supreme Court ruled that the state of Oregon could bar the use of mescaline in Native American religious ceremonies. The Religious Freedom Restoration Act (RFRA) in 1993 allowed the use of peyote in religious ceremony but in 1997, the Supreme Court ruled that the RFRA was unconstitutional when applied against states. However, in a subsequent case, the Court held that the Government had not carried the burden under the Religious Freedom Restoration Act of showing a compelling interest that allowed no exception to the ban on the use of the drug to accommodate a sincere religious practice.[19] Thus, the current state of the law is that, while the federal government may not restrict use of peyote in ceremony, individual states do have a right to restrict its use.[20]
In the UK, mescaline in purified powder form is a Class A drug. However, dried cactus can be bought and sold legally, unlike raw "magic" (psilocybin containing) mushrooms, which are now illegal.[21] In Australia the peyote cacti and mescaline are strictly illegal, however San Pedro and other mescaline-containing plants are legal for ornamental/gardening purposes.[citation needed] In Canada and Germany, mescaline in raw form and dried mescaline containing cacti are considered an illegal drug. However, anyone may grow and use peyote, or Lophophora williamsii, as well as San Pedro and San Peruvianus without restriction, as it is specifically exempt from legislation.[1]
See also
References
- ^ a b c Drug Identification Bible. Grand Junction, CO: Amera-Chem, Inc.. 2007. ISBN 0-9635626-9-X.
- ^ Chemistry of Acacia's from South Texas[dead link]
- ^ "Erowid Online Books : "PIHKAL" - #96 M". Erowid.org. http://www.erowid.org/library/books_online/pihkal/pihkal096.shtml. Retrieved 2011-09-07.
- ^ AJ Giannini, AE Slaby, MC Giannini. Handbook of Overdose and Detoxification Emergencies. New Hyde Park, NY. Medical Examination Publishing/Excerpta Medica Company, 1982.
- ^ A James Giannini (1997). Drugs of Abuse (Second ed.). Los Angeles: Practice Management Information Corp. ISBN 1-57066-053-0.
- ^ Dewick, P. M. (2009). Medicinal Natural Products: A Biosynthetic Approach. United Kingdom: John Wiley & Sons. 335-336.
- ^ Smith, Michael Valentine. "Psychedelics and Society." Erowid.org. Web. 04 Dec. 2010. <http://www.erowid.org/archive/rhodium/chemistry/psychedelicchemistry/chapter1.html>.
- ^ Wu D, Otton SV, Inaba T, Kalow W, Sellers EM (June 1997). "Interactions of amphetamine analogs with human liver CYP2D6". Biochem. Pharmacol. 53 (11): 1605–12. doi:10.1016/S0006-2952(97)00014-2. PMID 9264312. http://linkinghub.elsevier.com/retrieve/pii/S0006-2952(97)00014-2.
- ^ Cochin J, Woods LA, Seevers MH (February 1951). "The absorption, distribution and urinary excretion of mescaline in the dog". J. Pharmacol. Exp. Ther. 101 (2): 205–9. PMID 14814616. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=14814616.
- ^ a b Diaz, Jaime. How Drugs Influence Behavior. Englewood Cliffs: Prentice Hall, 1996
- ^ AJ Giannini, AE Slaby. Drugs of Abuse. Oradell, NJ. Medical Economics Books,1989, pp.207-239.
- ^ Nichols DE (February 2004). "Hallucinogens". Pharmacol. Ther. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ^ Monte AP, Waldman SR, Marona-Lewicka D, et al. (September 1997). "Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives". J. Med. Chem. 40 (19): 2997–3008. doi:10.1021/jm970219x. PMID 9301661.
- ^ Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R (June 2007). "Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex". Proc. Natl. Acad. Sci. U.S.A. 104 (23): 9870–5. doi:10.1073/pnas.0700436104. PMC 1887564. PMID 17535909. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1887564.
- ^ "Neuropharmacology of Hallucinogens". Erowid.org. 2009-03-27. http://www.erowid.org/psychoactives/pharmacology/pharmacology_article1.shtml. Retrieved 2011-09-07.
- ^ "Mescaline, serotonin and dopamine". Mescaline.com. http://mescaline.com/medsci/catsdopsero.html. Retrieved 2011-09-07.
- ^ United States Department of Justice. "Drug Scheduling". http://www.usdoj.gov/dea/pubs/scheduling.html. Retrieved 2007-11-02.
- ^ "List of psychotropic substances under international control". International Narcotics Control Board. http://www.incb.org/pdf/e/list/green.pdf. Retrieved 2008-01-27.
- ^ "Gonzales, Attorney General, Et Al. V. O Centro Espirita Beneficente Uniao Do Vegetal Et Al". Caselaw.lp.findlaw.com. http://caselaw.lp.findlaw.com/cgi-bin/getcase.pl?court=US&navby=case&vol=000&invol=04-1084. Retrieved 2011-09-07.
- ^ "Uses Drugs of Abuse—Origins and Effects - Hallucinogens". http://www.libraryindex.com/pages/2339/Drugs-Abuse-Origins-Uses-Effects-HALLUCINOGENS.html.
- ^ "2007 U.K. Trichocereus Cacti Legal Case Regina v. Saul Sette". http://www.erowid.org/plants/cacti/cacti_law2.shtml.
External links
- National Institutes of Health - National Institute on Drug Abuse Hallucinogen InfoFacts
- Mescaline at Erowid
- PiHKAL entry
- Mescaline entry in PiHKAL • info
- Mescaline: The Chemistry and Pharmacology of its Analogs, an essay by Alexander Shulgin
- Mescaline on the Mexican Border
Serotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolDrugs from PiHKAL AEM • AL • Aleph • Aleph-2 • Aleph-4 • Aleph-6 • Aleph-7 • Ariadne • Asymbescaline • Buscaline • Beatrice • Bis-TOM • BOB • BOD • BOH • BOHD • BOM • 4-Bromo-3,5-dimethoxyamphetamine • 2-Bromo-4,5-methylenedioxyamphetamine • 2C-B • 3C-BZ • 2C-C • 2C-D • 2C-E • 3C-E • 2C-F • 2C-G • 2C-G-3 • 2C-G-4 • 2C-G-5 • 2C-G-N • 2C-H • 2C-I • 2C-N • 2C-O • 2C-O-4 • 2C-P • CPM • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • psi-2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 4-D • beta-D • DESOXY • 2,4-DMA • 2,5-DMA • 3,4-DMA • DMCPA • DME • DMMDA • DMMDA-2 • DMPEA • DOAM • DOB • DOBU • DOC • DOEF • DOET • DOI • DOM • psi-DOM • DON • DOPR • Escaline • EEE • EEM • EME • EMM • Ethyl-J (EBDB) • Ethyl-K • F-2 • F-22 • Flea • G-3 • G-4 • G-5 • G-N • Ganesha • HOT-2 • HOT-7 • HOT-17 • IDNNA • Isomescaline • Isoproscaline • Iris • J (BDB) • Lophophine • PMA • Mescaline • Madam-6 • Methallylescaline • MDA • MDAL • MDBU • MDBZ • MDCPM • MDDM • MDE • MDHOET • MDIP • MDMA • MDMC (EDMA) • MDMEO • MDMEOET • MDMP • MDOH • MDPEA • MDPH • MDPL • MDPR • Metaescaline • MEDA • MEE • MEM • MEPEA • Meta-DOB • Meta-DOT • Methyl-DMA • Methyl-DOB • Methyl-J (MBDB) • Methyl-K • Methyl-MA (PMMA) • Methyl-MMDA-2 • MMDA • MMDA-2 • MMDA-3a • MMDA-3b • MME • Metaproscaline • MPM • Ortho-DOT • Proscaline • Phenescaline • Phenethylamine • Propynyl • Symbescaline • 2,3,4,5-Tetramethoxyamphetamine • 3-TASB • 4-TASB • 5-TASB • Thiobuscaline • 3-TE • 4-TE • 3-TIM • 4-TIM • 5-TIM • 3-TM • 4-TM • TMA • TMA-2 • TMA-3 • TMA-4 • TMA-5 • TMA-6 • 3-TME • 4-TME • 5-TME • 2T-MMDA-3a • 4T-MMDA-2 • 2-TOET • 5-TOET • 2-TOM • 5-TOM • TOMSO • Thioproscaline • Trisescaline • 3-TSB • 4-TSB • 3-T-Trisescaline • 4-T-Trisescaline
Categories:- Entheogens
- Natural phenethylamine alkaloids
- Native American Church
- Psychedelic phenethylamines
- Serotonin receptor agonists
- Phenol ethers
Wikimedia Foundation. 2010.

