- Meisenheimer complex
-
A Meisenheimer complex or Jackson-Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene carrying electron withdrawing groups and nucleophile. These complexes are found as reactive intermediates in nucleophilic aromatic substitution but stable and isolated Meisenheimer salts are also known.[1][2][3]
Contents
Background
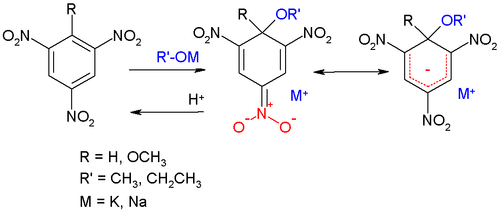
The early development of this type of complex takes place around the turn of the 19th century. In 1886 Janovski observed an intense violet color when he mixed meta-dinitrobenzene with an alcoholic solution of alkali. In 1895 Lobry de Bruyn investigated a red substance formed in the reaction of trinitrobenzene with potassium hydroxide in methanol. In 1900 Jackson and Gazzolo reacted trinitroanisole with sodium methoxide and proposed a quinoid structure for the reaction product.
In 1902 Jakob Meisenheimer [4] observed that by acidifying their reaction product, the starting material was recovered.
With three electron withdrawing groups, the negative charge in the complex is located at one of the nitro groups according to the quinoid model. When less electron poor arenes this charge is delocalized over the entire ring (structure to the right in scheme 1).
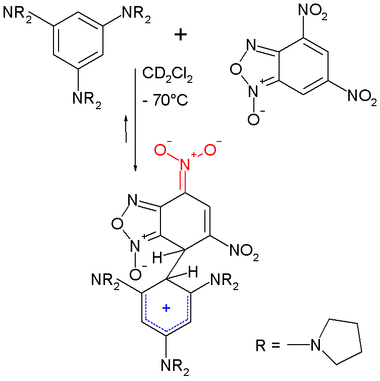
In one study[5] a Meisenheimer arene (4,6-Dinitrobenzofuroxan) was allowed to react with a strongly electron-releasing arene (1,3,5-tris(N-pyrrolidinyl)benzene) forming a zwitterionic Meisenheimer - Wheland complex. The Wheland intermediate is its opposite number and the reactive intermediate in electrophilic aromatic substitution.
The structure of this complex was confirmed by NMR spectroscopy.
Janovski reaction
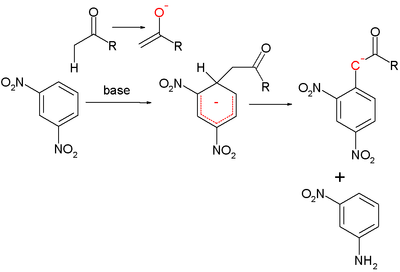
The Janovski reaction is the reaction of 1,3-dinitrobenzene with an enolizable ketone to the Meisenheimer adduct.
Zimmermann reaction
In the Zimmermann reaction the Janovski adduct is oxidized with excess base to a strongly colored enolate with subsequent reduction of the dinitro compound to the aromatic nitro amine.[6] This reaction is the basis of the Zimmermann test used for the detection of Ketosteroids.
References
- ^ G. A. Artamkina, M. P. Egorov, I. P. Beletskaya (1982). "Some aspects of anionic σ-complexes". Chem. Rev. 82 (4): 427–459. doi:10.1021/cr00050a004.
- ^ Francois Terrier (1982). "Rate and equilibrium studies in Jackson-Meisenheimer complexes". Chem. Rev. 82 (2): 77–152. doi:10.1021/cr00048a001.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Meisenheimer complex".
- ^ Jakob Meisenheimer (1902). "Ueber Reactionen aromatischer Nitrokörper". Justus Liebigs Annalen der Chemie 323 (2): 205–246. doi:10.1002/jlac.19023230205.
- ^ Carla Boga, Erminia Del Vecchio, Luciano Forlani, Andrea Mazzanti, Paolo E. Todesco (2005). "Evidence for Carbon-Carbon Meisenheimer-Wheland Complexes between Superelectrophilic and Supernucleophilic Carbon Reagents". Angewandte Chemie 117 (21): 3349–3353. doi:10.1002/ange.200500238.
- ^ Rolf Gleiter, Karl Heinz Pfeifer, Guenter Szeimies, Johannes Belzner, and Klaus Lehne (1990). "Electronic structure of [n.1.1]propellanes. PE (photoelectron) spectroscopic investigations". J. Org. Chem. 55 (2): 633–636. doi:10.1021/jo00289a044.
Categories:- Reactive intermediates
- Salts
Wikimedia Foundation. 2010.