- Acetone peroxide
-
Acetone peroxide 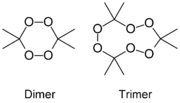

 3,3,6,6-Tetramethyl-1,2,4,5-tetraoxane
3,3,6,6-Tetramethyl-1,2,4,5-tetraoxane
(dimer)
3,3,6,6,9,9-Hexamethyl-1,2,4,
5,7,8-hexaoxacyclononane
(trimer)Identifiers CAS number 17088-37-8 
PubChem 536100 ChemSpider 3582942 
Jmol-3D images Image 1
Image 2- CC1(C)OOC(C)(C)OO1 (dimer)
CC1(C)OOC(C)(C)OOC(C)(C)OO1 (trimer)
CC1(OOC(OOC(OO1)(C)C)(C)C)C
Properties Molecular formula C6H12O4 (dimer)
C9H18O6 (trimer)Molar mass 148.157 g/mol (dimer)
222.24 g/mol (trimer)Appearance White crystalline solid Melting point 91 °C, 364 K, 196 °F
Boiling point 97–160 °C
Solubility in water insoluble Hazards Main hazards Explosive Explosive data Shock sensitivity High / moderate when wet Friction sensitivity High / moderate when wet Explosive velocity 5300 m/s
17,384 ft/s
3.29 miles per secondRE factor .83  peroxide (verify) (what is:
peroxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Acetone peroxide (triacetone triperoxide, peroxyacetone, TATP, TCAP) is an organic peroxide and a primary high explosive. It takes the form of a white crystalline powder with a distinctive bleach-like odor.
It is susceptible to heat, friction, and shock. The instability is greatly altered by impurities, including its own oligomers. It is normally fairly stable when pure trimer[citation needed]. It is not easily soluble in water. It is more stable and less sensitive when wet.
Contents
History
Acetone peroxide was discovered in 1895 by Richard Wolffenstein.[1][2] He was the first chemist to use inorganic acids as catalysts. He was also the first researcher to receive a patent for using the peroxide as an explosive compound. In 1900 Bayer and Villiger described in the same journal the first synthesis of the dimer and also described use of acids for the synthesis of both peroxides.[3] Information about these procedures including the relative proportions of monomer, dimer, and trimer is also available in an article by Milas and Golubović.[4] Other sources include crystal structure and 3d analysis in The Chemistry of Peroxides edited by Saul Patai (pp. 396–7), as well as the Textbook of Practical Organic Chemistry by Vogel.
Chemistry
"Acetone peroxide" most commonly refers to the cyclic trimer TCAP (tri-cyclic acetone peroxide, or tri-cyclo, C9H18O6) obtained by a reaction between hydrogen peroxide and acetone in an acid-catalyzed nucleophilic addition.[5] The dimer (C6H12O4) and open monomer are also formed, but under proper conditions the cyclic trimer is the primary product. A tetrameric form was also described.[6] In mildly acidic or neutral conditions, the reaction is much slower and produces more monomeric organic peroxide than the reaction with a strong acid catalyst. Due to significant strain of the chemical bonds in the dimer and especially the monomer, they are even more unstable than the trimer.[7]
At room temperature, the trimeric form slowly sublimes, reforming as larger crystals of the same peroxide.
Acetone peroxide is notable as a high explosive not containing nitrogen. This is one reason why it has become popular with terrorists,[8] as it can pass through scanners designed to detect nitrogenous explosives.[9]
TCAP generally burns when ignited, unconfined, in quantities less than about 4 grams. More than 4 grams will usually detonate when ignited; smaller quantities might detonate when even slightly confined. Completely dry TCAP is much more prone to detonation than the fresh product still wetted with water or acetone. The oxidation that occurs when burning is:
- 2 C9H18O6 + 21 O2 → 18 H2O + 18 CO2
Theoretical examination of the explosive decomposition of TCAP, in contrast, predicts "formation of acetone and ozone as the main decomposition products and not the intuitively expected oxidation products."[10] This result is in good agreement with the results of 60 years of the study of controlled decompositions in various organic peroxides. It is the rapid creation of gas from a solid that creates the explosion. Very little heat is created by the explosive decomposition of TCAP. Recent research describes TCAP decomposition as an entropic explosion.[10]
The high sensitivity to shock, heat and friction are due to the instability of the molecule. Big crystals, found in older mixtures, are more dangerous, as they are easier to shatter—and initiate—than small ones.
Due to the low cost and ease with which the precursors can be obtained, acetone peroxide can be manufactured by those without the resources needed to manufacture or buy more sophisticated explosives. When the reaction is carried out without proper equipment the risk of an accident is significant. Simply mixing sulfuric acid, hydrogen peroxide, and acetone can create the substance.[citation needed] Crystals of AP soon precipitate out.
There is a common myth that the only "safe" acetone peroxide is the trimer, made at low temperatures:
"The mixture must be kept below 10 degrees Celsius. If the crystals form at this temperature, it forms the isomer called tricycloacetone peroxide, which is relatively stable and safe to handle. If the crystals form above this temperature, the dimeric form, called dicycloacetone peroxide. This isomer is much more unstable, and could go off at the touch, making it not safe enough to be considered a practical explosive. As long as the temperature is kept below 10 degrees Celsius, then there is little to worry about."[11]
The trimer is the more stable form, but is not much more stable than the dimer. All forms of acetone peroxide are sensitive to initiation. Organic peroxides are sensitive, dangerous explosives; due to their sensitivity they are rarely used by well funded militaries. Even for those who synthesize explosives as a hobby there are far safer explosives with syntheses nearly as simple as that of acetone peroxide.
Acetone peroxide is commonly combined with nitrocellulose by dissolving the nitrocellulose in acetone and then mixing in the acetone peroxide and letting it dry, this results in a mixture that is both more stable and somewhat more powerful than acetone peroxide by itself. This mixture is commonly referred to as APNC.
Tetrameric acetone peroxide is more chemically stable (heating to 120°C for 4 hours), but despite this, it is still a very dangerous primary explosive. It can be prepared using tin(IV) chloride (without acid present) as a catalyst with up to 40% yield if radical inhibitor such as hydroquinone, or a chelator such as EDTA is added.[6]
It evaporates 6.5% in 24 h at 14–18°C. Openly at air at 25°C has a loss by sublimation of 68.6% in 14 days.[12] Many accidents resulted from the fact that acetone peroxide detonates due to its sublimation characteristics within fewer days by crystallization in the range of the container cap, when opening the same. Keeping it wet stops the sublimation and can prevent this type of accident.
Industrial occurrence
Acetone peroxides are common and unwanted by-products of oxidation reactions, such as those used in phenol syntheses. Due to their explosivity, they are hazardous. Numerous methods are used to reduce their production – shifting the pH to more alkaline, adjusting the reaction temperature, or adding a soluble copper(II) compound.[13]
Acetone peroxide and benzoyl peroxide are used as flour bleaching agents to bleach and "mature" flour.[14]
Ketone peroxides, including acetone peroxide, methyl ethyl ketone peroxide, and benzoyl peroxide, find applications as initiators for polymerization reactions of e.g. silicone or polyester resins, often encountered when making fiberglass-reinforced composites. For these uses, the peroxides are typically in the form of a dilute solution in an organic solvent, though even commercial products with higher concentrations of organic peroxides can form crystals around the lid when older, making the can shock-sensitive. Methyl ethyl ketone is more common for this purpose, as it is stable in storage.
Accidental byproduct
Acetone peroxide can also occur accidentally, when suitable chemicals are mixed together, for example when methyl ethyl ketone peroxide is mixed with acetone while making fiberglass composites, and left to stand for some time, or when a mixture of peroxide and hydrochloric acid from printed circuit board etching is mixed with waste acetone from cleaning the finished board and allowed to stand. While amounts obtained this way are typically much smaller than from intentional production, they are also less pure and prepared without cooling, and hence very unstable.
It is also a hazardous by-product of isosafrole oxidation in acetone, a step in the synthesis of MDMA.
Use in improvised explosive devices
See also: Improvised explosive deviceTATP is relatively easy to make and has been used in suicide attacks and in improvised explosive devices.[15] [16] Due to its high susceptibility to accidental detonation by shock, friction, or sparks, acetone peroxide has earned the nickname "Mother of Satan" among certain Islamist militant groups.[8]
References
- ^ Wolffenstein, R (1895). "Über die Einwirkung von Wasserstoffsuperoxyd auf Aceton und Mesityloxyd (On the effect of hydrogen peroxide on acetone and mesityl oxide)". Chemische Berichte 28 (2): 2265. doi:10.1002/cber.189502802208.
- ^ See also: Richard Wolffenstein, Deutsches Reich Patent 84,953 (1895).
- ^ Baeyer, Adolf; Villiger, Victor (1900). "Über die Einwirkung des Caro'schen Reagens auf Ketone [On the effect of Caro's reagent on ketones]". Berichte der deutschen chemischen Gesellschaft 33: 858–864. doi:10.1002/cber.190003301153.; See also Baeyer, Adolf; Villiger, Victor (1900). "Über die Nomenclatur der Superoxyde und die Superoxyde der Aldehyde". Berichte der deutschen chemischen Gesellschaft 33 (2): 2479–2487. doi:10.1002/cber.190003302185.
- ^ Milas N. A., Golubović A. (1959). "Studies in Organic Peroxides. XXVI. Organic Peroxides Derived from Acetone and Hydrogen Peroxide". Journal of the American Chemical Society 81 (24): 6461–6462. doi:10.1021/ja01533a033.
- ^ "Megalomania's Method of Making Acetone Peroxide". Megalomania's Controversial Chem Lab. January 31, 2004. http://www.roguesci.org/megalomania/explo/acetoneperoxide.html.
- ^ a b Jiang H., Chu G., Gong H., Qiao Q. (1999). "Tin Chloride Catalysed Oxidation of Acetone with Hydrogen Peroxide to Tetrameric Acetone Peroxide". Journal of Chemical Research 28 (4): 288–289. doi:10.1039/a809955c.
- ^ Schulte-Ladbeck, R.; Kolla, P.; Karst, U. (2003). "Trace Analysis of Peroxide-Based Explosives". Analytical Chemistry 75 (4): 731–735. doi:10.1021/ac020392n. PMID 12622359.
- ^ a b Genuth, Iddo; Lucille Fresco-Cohen (6 November 2006). "TATP: Countering the Mother of Satan". The Future of Things. http://thefutureofthings.com/articles/35/tatp-countering-the-mother-of-satan.html. Retrieved 24 September 2009. "The tremendous devastative force of TATP, together with the relative ease of making it, as well as the difficulty in detecting it, made TATP one of the weapons of choice for terrorists"
- ^ "Feds are all wet on airport security". Star-Ledger. 24 August 2006. http://results.factiva.com/index/index.aspx?ref=NSL0000020060824e28o0001y. Retrieved 11 September 2009. "At the moment, Watts said, the screening devices are set to detect nitrogen-based explosives, a category that doesn't include TATP"
- ^ a b F. Dubnikova, R. Kosloff, J. Almog, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt, E. Keinan (2003). "Decomposition of Triacetone Triperoxide Is an Entropic Explosion". Journal of the American Chemical Society 127 (4): 1146–1159. doi:10.1021/ja0464903. PMID 15669854. http://www.technion.ac.il/~keinanj/pub/122.pdf.
- ^ "Detailed Description of the Synthesis of Acetone Peroxide". totse.com. http://www.totse.info/en/bad_ideas/ka_fucking_boom/162660.html. Retrieved May 29, 2010.
- ^ Acetone peroxide / Explosive. Economypoint.org. Retrieved on 2010-12-13.
- ^ Destruction of acetone peroxide patent
- ^ Food additives list from the codex alimentarius
- ^ July 15, 2005 TimesOnline
- ^ Yuen-C, Tham (16 July 2011). "Fighting terror takes new thinking: DPM Teo". The Strait Times. http://www.straitstimes.com/BreakingNews/Singapore/Story/STIStory_691320.html. Retrieved 7 August 2011.
External links
Categories:- Explosive chemicals
- Ketals
- Organic peroxides
- Oxygen heterocycles
- Radical initiators
- CC1(C)OOC(C)(C)OO1 (dimer)
Wikimedia Foundation. 2010.
