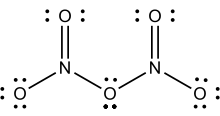
- Dinitrogen pentoxide
-
Dinitrogen pentoxide 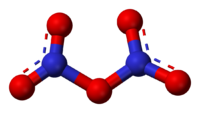
 Dinitrogen pentaoxideOther namesNitric acid anhydride
Dinitrogen pentaoxideOther namesNitric acid anhydride
dnpoIdentifiers CAS number 10102-03-1 
PubChem 66242 ChemSpider 59627 
ChEBI CHEBI:29802 
Jmol-3D images Image 1 - [O-][N+](=O)O[N+]([O-])=O
Properties Molecular formula N2O5 Molar mass 108.01 g/mol Appearance white solid Density 1.642 g/cm3 (18 °C) Melting point 41 °C[1]
Boiling point 47 °C (sublimates)
Solubility in water reacts to give HNO3 Solubility soluble in chloroform Structure Molecular shape planar, C2v (approx. D2h)
N–O–N ≈ 180ºThermochemistry Std enthalpy of
formation ΔfHo298−43.1 kJ/mol (s)
+11.3 kJ/mol (g)Standard molar
entropy So298178.2 J K−1 mol−1 (s)
355.6 J K−1 mol−1 (g)Hazards EU Index Not listed Main hazards strong oxidizer, forms strong acid in contact with water NFPA 704 Flash point Non-flammable Related compounds Related nitrogen oxides Nitrous oxide
Nitric oxide
Dinitrogen trioxide
Nitrogen dioxide
Dinitrogen tetroxideRelated compounds Nitric acid  pentoxide (verify) (what is:
pentoxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dinitrogen pentoxide is the chemical compound with the formula N2O5. Also known as nitrogen pentoxide, N2O5 is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by NO2BF4 (nitronium tetrafluoroborate).
N2O5 is a rare example of a compound that adopts two structures depending on the conditions: most commonly it is a salt, but under some conditions it is a polar molecule:
- N2O5 ⇌ [NO2+][NO3−]
Contents
Syntheses and properties
N2O5 was first reported by Deville in 1840, who prepared it by treating AgNO3 with Cl2.[2][3] A recommended laboratory synthesis entails dehydrating nitric acid (HNO3) with phosphorus(V) oxide:[4]
- P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5
In the reverse process, N2O5 reacts with water (hydrolyses) to produce nitric acid. Thus, nitrogen pentoxide is the anhydride of nitric acid:
- N2O5 + H2O → 2 HNO3
N2O5 exists as colourless crystals that sublime slightly above room temperature. The salt eventually decomposes at room temperature into NO2 and O2. [5]
Structure
Solid N2O5 is a salt, consisting of separated anions and cations. The cation is the linear nitronium ion NO2+ and the anion is the planar nitrate NO3− ion. Thus, the solid could be called nitronium nitrate. Both nitrogen centers have oxidation state +5.
The intact molecule O2N–O–NO2 exists in the gas phase (obtained by subliming N2O5) and when the solid is extracted into nonpolar solvents such as CCl4. In the gas phase, the O–N–O angle is 133° and the N–O–N angle is 114°. When gaseous N2O5 is cooled rapidly ("quenched"), one can obtain the metastable molecular form, which exothermically converts to the ionic form above −70 °C.[4]
Reactions and applications
Dinitrogen pentoxide, for example as a solution in chloroform, has been used as a reagent to introduce the NO2 functionality. This nitration reaction is represented as follows:
- N2O5 + Ar–H → HNO3 + Ar–NO2
where Ar represents an arene moiety.
N2O5 is of interest for the preparation of explosives.[3][6]
NO2BF4
Replacement of the NO3− portion of N2O5 with BF4− gives NO2BF4 (CAS#13826-86-3). This salt retains the high reactivity of NO2+, but it is thermally stable, decomposing at ca. 180°C (into NO2F and BF3). NO2BF4 has been used to nitrate a variety of organic compounds, especially arenes and heterocycles. Interestingly, the reactivity of the NO2+ can be further enhanced with strong acids that generate the "super-electrophile" HNO22+.
Hazards
N2O5 is a strong oxidizer that forms explosive mixtures with organic compounds and ammonium salts. The decomposition of dinitrogen pentoxide produces the highly toxic nitrogen dioxide gas.
References
- ^ Emeleus (1 January 1964). Advances in Inorganic Chemistry. Academic Press. pp. 77–. ISBN 978-0-12-023606-0. http://books.google.com/books?id=UPBKxgY20lEC&pg=PA77. Retrieved 20 September 2011.
- ^ M.H. Deville (1849). "Note sur la production de l'acide nitrique anhydre". Compt. Rend. Acad. Sci. Paris 28: 257–260. http://www.archive.org/details/comptesrendusheb28acad.
- ^ a b Jai Prakash Agrawal (19 April 2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley-VCH. pp. 117–. ISBN 978-3-527-32610-5. http://books.google.com/books?id=rqZROysoS7QC&pg=PA117. Retrieved 20 September 2011.
- ^ a b Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5
- ^ Nitrogen(V) Oxide. Inorganic Syntheses. 3. 1950. pp. 78–81.
- ^ Talawar, M. B. et al. (2005). "Establishment of Process Technology for the Manufacture of Dinitrogen Pentoxide and its Utility for the Synthesis of Most Powerful Explosive of Today—CL-20". Journal of Hazardous Materials 124: 153–64. doi:10.1016/j.jhazmat.2005.04.021.
Categories:- Oxides
- Inorganic nitrogen compounds
- Acid anhydrides
- Acidic oxides
Wikimedia Foundation. 2010.