- Norethisterone
-
Norethisterone 
Systematic (IUPAC) name (17β)-17-ethynyl-17-hydroxyestr-4-en-3-one;
(8R,9S,10R,13S,14S,17S)-17-ethynyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-oneClinical data AHFS/Drugs.com International Drug Names MedlinePlus a604034 Pregnancy cat. ? Legal status ? Pharmacokinetic data Bioavailability 64% Protein binding >95% Half-life 7 hours Identifiers CAS number 68-22-4 
ATC code G03AC01 G03DC02 PubChem CID 6230 DrugBank APRD00679 ChemSpider 5994 
UNII T18F433X4S 
KEGG D00182 
ChEBI CHEBI:7627 
ChEMBL CHEMBL1162 
Chemical data Formula C20H26O2 Mol. mass 298.419 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
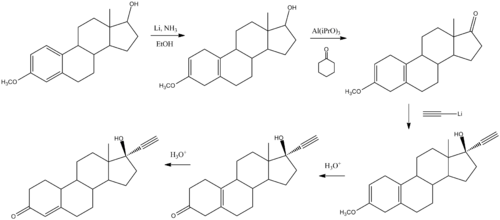
(what is this?) (verify)Norethisterone (or norethindrone) (or 19-nor-17α-ethynyltestosterone) is a molecule used in some combined oral contraceptive pills, progestogen only pills and is also available as a stand-alone drug. It is a progestogen and can be used to treat premenstrual syndrome, painful periods, abnormal heavy bleeding, irregular periods, menopausal syndrome (in combination with oestrogen), or to postpone a period. It is also commonly used to help prevent uterine hemorrhage in complicated non-surgical or pre-surgical gynecologic cases. Norethindrone was the first orally highly active progestin to be synthesized. It was synthesized for the first time by chemists Luis Miramontes, Carl Djerassi, and George Rosenkranz at Syntex in Mexico City in 1951.[1] It was the progestin used in one of the first two oral contraceptives. It is often used as the related ester, norethisterone acetate.
Chemistry
References
- ^ Djerassi, C.; Miramontes, L.; Rosenkranz, G.; Sondheimer, Franz (1954). "Steroids. LIV. Synthesis of 19-Nor-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone". J. Am. Chem. Soc. 76 (16): 4092–94. doi:10.1021/ja01645a010.
Estrogens and progestogens (G03C-D, L02) Progestogens/
progestins
(progesterone)AgonistAndrostene (Drospirenone) • 19-norprogesterone (Nomegestrol • Promegestone • Trimegestone) • 19-nortestosterone (Dienogest)Other/
ungroupedPregnenedione (Gestonorone) • Pregnene (Ethisterone) • Pregnadiene (Medrogestone • Melengestrol) • Norpregnane (Norgestrienone) • Lynestrenol • Norethynodrel • Tibolone • Dydrogesterone • Quingestanolantagonist: MifepristoneAsoprisnil • CDB-4124 • Ulipristal acetateEstrogens AgonistDiosgenin • Estradiol (Ethinylestradiol#/Mestranol • Estradiol 17 beta-cypionate# • Polyestradiol phosphate) • Estrone (Estrone sulfate) • Estriol • Promestriene • Equilenin • EquilinAfimoxifene • Arzoxifene • Bazedoxifene • Cyclofenil • Lasofoxifene • Ormeloxifene • Raloxifene • Tamoxifen • Toremifenepure antagonist: Fulvestrant
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it.