- Ammonium perchlorate
-
Ammonium perchlorate 
 Ammonium perchlorateOther namesAP
Ammonium perchlorateOther namesAPIdentifiers CAS number 7790-98-9 
ChemSpider 23041 
EC number 232-235-1 UN number 1442 RTECS number SC7520000 Jmol-3D images Image 1 - [O-]Cl(=O)(=O)=O.[NH4+]
Properties Molecular formula NH4ClO4 Molar mass 117.49 g/mol Appearance white granular Density 1.95 g/cm3 Melting point Exothermic decomposition before melting at >200 °C[1]
Solubility in water 11.56 g/100 mL (0 °C)
20.85 g/100 mL (20 °C)
57.01 g/100 mL (100 °C)Solubility soluble in methanol
partially soluble in acetone
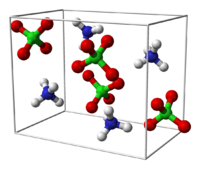
insoluble in etherStructure Crystal structure Orthorhombic (< 513 K)
Cubic (> 513 K)Hazards MSDS External MSDS EU Index 017-009-00-0 EU classification Oxidant (O) R-phrases R9, R44 S-phrases (S2), S14, S16, S27, S36/37 NFPA 704 Related compounds Other anions Ammonium chlorate
Ammonium chlorideOther cations Potassium perchlorate
Sodium perchlorate
Lithium perchlorateRelated compounds Perchloric acid  perchlorate (verify) (what is:
perchlorate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ammonium perchlorate is an inorganic compound with the formula NH4ClO4. It is the salt of perchloric acid and ammonia. It is a powerful oxidizer, which is why its main use is in solid propellants. It has been implicated in a number of industrial accidents.
Contents
Production
Ammonium perchlorate (AP) is produced by reaction between ammonia and perchloric acid, and is the driver behind the industrial production of perchloric acid. It also can be prepared by treatment of ammonium salts with sodium perchlorate. This process exploits the fact that the solubility of NH4ClO4 is about 10% of that for sodium perchlorate.[2]
AP crystallises into colorless rhombohedra.
Decomposition
Like most ammonium salts, ammonium perchlorate decomposes before melting. Mild heating results in the evolution of chlorine, nitrogen, oxygen, and water.
- 2 NH4ClO4 → Cl2 + N2 + 2 O2 + 4 H2O
The combustion of AP is quite complex and is widely studied. AP crystals decompose before melting, even though a thin liquid layer has been observed on crystal surfaces during high-pressure combustion processes.[3] Strong heating may lead to explosions. Complete reactions leave no residue. Pure crystals cannot sustain a flame below the pressure of 20 bar (2 MPa).
AP is a Class 4 oxidizer (can undergo an explosive reaction) for particle sizes over 15 micrometres[4] and is classified as an explosive for particle sizes less than 15 micrometres.[5][6]
Applications
The vast majority of ammonium perchlorate is used to make solid propellants.[7] When AP is mixed with a fuel (like a powdered aluminum and/or with an elastomeric binder), it can generate self-sustained combustion at far under atmospheric pressure. It is an important oxidizer with a decades-long history of use in solid rocket propellants — space launch (including the Space Shuttle Solid Rocket Booster), military, amateur, and hobby high-power rockets, as well as in some fireworks.
Some "breakable" epoxy adhesives contain suspensions of AP. Upon heating to 300 °C, the AP degrades the organic adhesive, breaking the cemented joint.
Toxicity
Perchlorate itself confers little acute toxicity. For example, sodium perchlorate has an LD50 of 2-4 g/kg and is eliminated rapidly after ingestion.[2] However, chronic exposure to perchlorates, even in low concentrations, has been shown to cause various thyroid problems, as it is taken up in place of iodine.
See also
References
- ^ Liu, L.; Li, F.; Tan, L.; Ming, L.; Yi, Y. (2004), "Effects of Nanometer Ni, Cu, Al and NiCu Powders on the Thermal Decomposition of Ammonium Perchlorate", Propellant, Explosives, Pyrotechnics 29: 34–38, doi:10.1002/prep.200400026
- ^ a b Helmut Vogt, Jan Balej, John E. Bennett, Peter Wintzer, Saeed Akbar Sheikh, Patrizio Gallone “Chlorine Oxides and Chlorine Oxygen Acids” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH. doi:10.1002/14356007.a06_483
- ^ T. L. Boggs, Deflagration Rate, Surface Structure and Subsurface Profile of Self-Deflagrating Single Crystals of Ammonium Perchlorate. AIAA Journal, 8(5), 1970, pp. 867--873.
- ^ NFPA 400: Hazardous Materials Code, 2010
- ^ NFPA 495: Explosive Materials Code, 2010
- ^ "Development of an Enhanced Hazard Classification System for Oxidizers Research Project, Technical Report", Safety Engineering Laboratories , Inc., The Fire Protection Research Foundation, 13 April 2006
- ^ "Perchlorate: Overview of Issues, Status, and Remedial Actions", ITRC, September 2005 accessed 4 July 2011
Categories:- Ammonium compounds
- Perchlorates
- Pyrotechnic oxidizers
- Rocket oxidizers
- Oxidizing agents
Wikimedia Foundation. 2010.

