- Lysine
-
Lysine 
 LysineOther names2,6-diaminohexanoic acid
LysineOther names2,6-diaminohexanoic acidIdentifiers CAS number 70-54-2 DL  , 56-87-1 L, 923-27-3 D
, 56-87-1 L, 923-27-3 DPubChem 866 ChemSpider 843  , 5747 L
, 5747 LKEGG C16440 
ChEBI CHEBI:25094 
ChEMBL CHEMBL28328 
IUPHAR ligand 724 Jmol-3D images Image 1 - C(CCN)CC(C(=O)O)N
Properties Molecular formula C6H14N2O2 Molar mass 146.19 g mol−1 Solubility in water 1.5kg/L @ 25 °C[1] Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Lysine (abbreviated as Lys or K)[2] is an α-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG.
Lysine is a base, as are arginine and histidine. The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. (The ε-amino group, which is attached to the NH3+ group, is the fifth carbon down from the α-carbon, which is attached to the carboxyl (C=OOH) group.[3])
Common posttranslational modifications include methylation of the ε-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications at lysine residues include acetylation and ubiquitination. Collagen contains hydroxylysine, which is derived from lysine by lysyl hydroxylase. O-Glycosylation of hydroxylysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
Contents
Biosynthesis
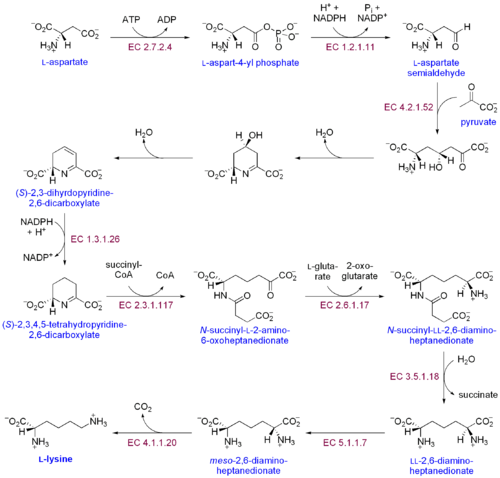
As an essential amino acid, lysine is not synthesized in animals, hence it must be ingested as lysine or lysine-containing proteins. In plants and bacteria, it is synthesized from aspartic acid (aspartate):[4]
- L-aspartate is first converted to L-aspartyl-4-phosphate by aspartokinase (or Aspartate kinase). ATP is needed as an energy source for this step.
- β-Aspartate semialdehyde dehydrogenase converts this into β-aspartyl-4-semialdehyde (or β-aspartate-4-semialdehyde). Energy from NADPH is used in this conversion.
- Dihydrodipicolinate synthase adds a pyruvate group to the β-aspartyl-4-semialdehyde, and two water molecules are removed. This causes cyclization and gives rise to 2,3-dihydrodipicolinate.
- This product is reduced to 2,3,4,5-tetrahydrodipicolinate (or Δ1-piperidine-2,6-dicarboxylate, in the figure: (S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate) by dihydrodipicolinate reductase. This reaction consumes a NADPH molecule.
- Tetrahydrodipicolinate N-acetyltransferase opens this ring and gives rise to N-succinyl-L-2-amino-6-oxoheptanedionate (or N-acyl-2-amino-6-oxopimelate). Two water molecules and one acyl-CoA (succinyl-CoA) enzyme are used in this reaction.
- N-succinyl-L-2-amino-6-oxoheptanedionate is converted into N-succinyl-LL-2,6-diaminoheptanedionate (N-acyl-2,6-diaminopimelate). This reaction is catalyzed by the enzyme succinyl diaminopimelate aminotransferase. A glutaric acid molecule is used in this reaction and an oxoacid is produced as a byproduct.
- N-succinyl-LL-2,6-diaminoheptanedionate (N-acyl-2,6-diaminopimelate)is converted into LL-2,6-diaminoheptanedionate (L,L-2,6-diaminopimelate) by succinyl diaminopimelate desuccinylase (acyldiaminopimelate deacylase). A water molecule is consumed in this reaction and a succinate is produced a byproduct.
- LL-2,6-diaminoheptanedionate is converted by diaminopimelate epimerase into meso-2,6-diamino-heptanedionate (meso-2,6-diaminopimelate).
- Finally, meso-2,6-diamino-heptanedionate is converted into L-lysine by diaminopimelate decarboxylase.
Enzymes involved in this biosynthesis include:[5]
- Aspartokinase
- β-Aspartate semialdehyde dehydrogenase
- Dihydropicolinate synthase
- Δ1-Piperidine-2,6-dicarboxylate dehydrogenase
- N-succinyl-2-amino-6ketopimelate synthase
- Succinyl diaminopimelate aminotransferase
- Succinyl diaminopimelate desuccinylase
- Diaminopimelate epimerase
- Diaminopimelate decarboxylase.
Metabolism
Lysine is metabolised in mammals to give acetyl-CoA, via an initial transamination with α-ketoglutarate. The bacterial degradation of lysine yields cadaverine by decarboxylation.
Allysine is a derivative of lysine, used in the production of elastin and collagen. It is produced by the actions of the enzyme lysyl oxidase on lysine in the extracellular matrix and is essential in the crosslink formation that stabilizes collagen and elastin.
Synthesis
Synthetic, racemic lysine has long been known.[6] A practical synthesis starts from caprolactam.[7] Industrially, L-lysine is usually manufactured by a fermentation process using Corynebacterium glutamicum; production exceeds 600,000 tons a year.[8]
Dietary sources
The nutritional requirement per day, in milligrams of lysine per kilogram of body weight, is: infants (3–4 months) 103, children (2 years) 64, older children (10–12 years) 60 to 44, adults 12.[9] For a 70 kg adult, 12 milligrams of lysine per kilogram of body weight is 0.84 grams of lysine. Good sources of lysine are foods rich in protein such as soy, as well as meat (specifically red meat, lamb, pork, and poultry), cheese (particularly Parmesan), certain fish (such as cod and sardines), and eggs.[10]
Lysine is the limiting amino acid (the essential amino acid found in the smallest quantity in the particular foodstuff) in most cereal grains, but is plentiful in most pulses (legumes).[11] Consequently, meals that combine cereal grains and legumes, such as the Indian dal with rice, Middle Eastern hummus, ful medames, falafel with pita bread, the Mexican beans with rice or tortilla have arisen to provide complete protein in diets that are, by choice or by necessity, vegetarian. A food is considered to have sufficient lysine if it has at least 51 mg of lysine per gram of protein (so that the protein is 5.1% lysine).[12]
Foods containing significant amounts of lysine include:
- Catfish, channel, farmed, raw: 9.19% of the protein is lysine.[13]
- Chicken, roasting, meat and skin, cooked, roasted: 8.11% of the protein is lysine.[14]
- Beef, ground, 90% lean/10% fat, cooked: 8.31% of the protein is lysine.[15]
- Soybean, mature seeds, raw: 7.42% of the protein is lysine.[16]
- Soybean, mature seeds, sprouts: 5.74% of the protein is lysine (sprouting decreases the lysine content).[17]
- Winged Bean (aka Goa Bean or Asparagus Pea), mature seeds, raw: 7.20% of the protein is lysine.[18]
- Lentil, pink, raw: 6.97% of the protein is lysine.[19]
- Lentil, sprouts, raw: 7.95% of the protein is lysine (sprouting increases the lysine content).[20]
- Parmesan cheese, grated: 7.75% of the protein is lysine.[21]
- Azuki bean (adzuki beans), mature seeds, raw: 7.53% of the protein is lysine.[22]
- Milk, non-fat: 7.48% of the protein is lysine.[23]
- Egg (food), whole, raw: 7.27% of the protein is lysine.[24]
- Pea, split, mature seeds, raw: 7.22% of the protein is lysine.[25]
- Kidney Bean, mature seeds, raw: 6.87% of the protein is lysine.[26]
- Chickpea, (garbanzo beans, bengal gram), mature seeds, raw: 6.69% of the protein is lysine.[27]
- Navy Bean, mature seeds, raw: 5.73% of the protein is lysine.[28]
- Amaranth, grain, uncooked: 5.17% of the protein is lysine.[29]
Properties
L-Lysine is a necessary building block for all protein in the body. L-Lysine plays a major role in calcium absorption; building muscle protein; recovering from surgery or sports injuries; and the body's production of hormones, enzymes, and antibodies.
Modifications
Lysine can be modified through acetylation, methylation, ubiquitination, sumoylation, neddylation, biotinylation, pupylation, and carboxylation, which tends to modify the function of the protein of which the modified lysine residue(s) are a part.[30]
Clinical significance
It has been suggested that lysine may be beneficial for those with herpes simplex infections.[31] However, more research is needed to fully substantiate this claim. For more information, refer to Herpes simplex - Lysine.
Lysine has a known anxiolytic action through its effects on serotonin receptors in the intestinal tract. One study on rats[32] showed that overstimulation of the 5-HT4 receptors in the gut are associated with anxiety-induced intestinal pathology. Lysine, acting as a serotonin antagonist and therefore reducing the overactivity of these receptors, reduced signs of anxiety and anxiety-induced diarrhea in the sample population. Another study showed that lysine deficiency leads to a pathological increase in serotonin in the amygdala, a brain structure that is involved in emotional regulation and the stress response.[33]
Human studies have also shown negative correlations between reduced lysine intake and anxiety. A population-based study in Syria included 93 families whose diet is primarily grain-based and therefore likely to be deficient in lysine. Fortification of grains with lysine was shown to reduce markers of anxiety, including cortisol levels, and also led to potentiation of benzodiazepine receptors (common targets of anxiolytic drugs such as Xanax and Ativan).[34]
There are lysine conjugates that show promise in the treatment of cancer, by causing cancerous cells to destroy themselves when the drug is combined with the use of phototherapy, while leaving non-cancerous cells unharmed.[35]
While chemically insignificant to lysine itself, it is worth noting that lysine is attached to dextroamphetamine to form the prodrug lisdexamfetamine (Vyvanse). In the gastrointestinal tract, the lysine molecule is cleaved from the dextroamphetamine, thereby making oral administration necessary.
According to animal studies, lysine deficiency causes immunodeficiency.[36] One cause of relative lysine deficiency is cystinuria, where there is impaired hepatic resorption of basic, or positively charged amino acids, including lysine. The accompanying urinary cysteine results because the same deficient amino acid transporter is normally present in the kidney as well.
Limited studies suggest that a high-lysine diet or L-lysine monochloride supplements may have a moderating effect on blood pressure and the incidence of stroke.[37]
Use of lysine in animal feed
Lysine production for animal feed is a major global industry, reaching in 2009 almost 700,000 tonnes for a market value of over €1.22 billion.[38] Lysine is an important additive to animal feed because it is a limiting amino acid when optimizing the growth of certain animals such as pigs and chickens for the production of meat. Lysine supplementation allows for the use of lower-cost plant protein (maize, for instance, rather than soy) while maintaining high growth rates, and limiting the pollution from nitrogen excretion.[39]
Lysine is industrially produced by microbial fermentation, from a base mainly of sugar. Genetic engineering research is actively pursuing bacterial strains to improve the efficiency of production and allow lysine to be made from other substrates.[38]
In popular culture
The 1993 film Jurassic Park, which is based on the 1990 Michael Crichton novel Jurassic Park, features dinosaurs that were genetically altered so that they could not produce lysine.[40] This was known as the "lysine contingency," and was supposed to prevent the cloned dinosaurs from surviving outside the park, forcing them to be dependent on lysine supplements provided by the park's veterinary staff. Most vertebrates cannot produce lysine by default (it is an essential amino acid).
In 1996, lysine became the focus of a price-fixing case, the largest in United States history. The Archer Daniels Midland Company paid a fine of US$100 million, and three of its executives were convicted and served prison time. Also found guilty in the price-fixing case were two Japanese firms (Ajinomoto, Kyowa Hakko) and a South Korean firm (Sewon).[41] Secret video recordings of the conspirators fixing lysine's price can be found online or by requesting the video from the U.S. Department of Justice, Antitrust Division. This case served as the basis of the movie The Informant!, and a book of the same title.[42]
See also
References
- ^ http://www.peptideguide.com/amino-acids/lysine.html
- ^ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. http://www.chem.qmul.ac.uk/iupac/AminoAcid/. Retrieved 2007-05-17.
- ^ Lysine. The Biology Project, Department of Biochemistry and Molecular Biophysics, University of Arizona.
- ^ Lysine biosynthesis and catabolism, Purdue University
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ Braun, J. V. “Synthese des inaktiven Lysins aus Piperidin" Berichte der deutschen chemischen Gesellschaft 1909, Volume 42, p 839-846. DOI: 10.1002/cber.190904201134.
- ^ Eck, J. C.; Marvel, C. S. “dl-Lysine Hydrochlorides” Organic Syntheses, Collected Volume 2, p.374 (1943). http://www.orgsyn.org/orgsyn/pdfs/CV2P0374.pdf
- ^ Pfefferle, W.; Möckel, B.; Bathe, B.; Marx, A. (2003). "Biotechnological manufacture of lysine". Advances in biochemical engineering/biotechnology. Advances in Biochemical Engineering/Biotechnology 79: 59–112. doi:10.1007/3-540-45989-8_3. ISBN 978-3-540-43383-5. PMID 12523389.
- ^ United Nations Food and Agriculture Organization: Agriculture and Consumer Protection. "Energy and protein requirements: 5.6 Requirements for essential amino acids". http://www.fao.org/DOCREP/003/AA040E/AA040E05.htm#ch5.6. Retrieved 2010-10-10.
- ^ University of Maryland Medical Center. "Lysine". http://www.umm.edu/altmed/articles/lysine-000312.htm. Retrieved 2009-12-30.
- ^ Young VR, Pellett PL (1994). "Plant proteins in relation to human protein and amino acid nutrition" (PDF). American Journal of Clinical Nutrition 59 (5 Suppl): 1203S–1212S. PMID 8172124. http://www.ajcn.org/content/59/5/1203S.long.
- ^ Institute of Medicine of the National Academies. "Dietary Reference Intakes for Macronutrients". http://www.iom.edu/Reports/2002/Dietary-Reference-Intakes-for-Energy-Carbohydrate-Fiber-Fat-Fatty-Acids-Cholesterol-Protein-and-Amino-Acids.aspx. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, catfish farmed". http://www.bitterpoison.com/protein/15234/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, chicken roasting meat skin". http://www.bitterpoison.com/protein/05112/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, beef ground 90 10 cooked". http://www.bitterpoison.com/protein/23563/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, soybean seeds". http://www.bitterpoison.com/protein/16108/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, soybeans sprouted". http://www.bitterpoison.com/protein/11452/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, winged bean seeds". http://www.bitterpoison.com/protein/16135/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, lentils". http://www.bitterpoison.com/protein/16144/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, lentils sprouted". http://www.bitterpoison.com/protein/11248/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, parmesan cheese". http://www.bitterpoison.com/protein/01032/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, adzuki bean". http://www.bitterpoison.com/protein/16001/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, milk nonfat". http://www.bitterpoison.com/protein/01085/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, egg whole". http://www.bitterpoison.com/protein/01123/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, pea split". http://www.bitterpoison.com/protein/16085/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, kidney bean". http://www.bitterpoison.com/protein/16032/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, chickpea". http://www.bitterpoison.com/protein/16056/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, navy bean". http://www.bitterpoison.com/protein/16037/. Retrieved 2010-10-10.
- ^ Helena Kloosterman; USDA National Nutrient Database for Standard Reference. "Essential Amino Acids Search, amaranth". http://www.bitterpoison.com/protein/20001/. Retrieved 2010-10-10.
- ^ Sadoul K, Boyault C, Pabion M, Khochbin S (February 2008). "Regulation of protein turnover by acetyltransferases and deacetylases". Biochimie 90 (2): 306–12. doi:10.1016/j.biochi.2007.06.009. PMID 17681659. http://linkinghub.elsevier.com/retrieve/pii/S0300-9084(07)00168-X.
- ^ Griffith RS, Norins AL, Kagan C. (1978). "A multicentered study of lysine therapy in Herpes simplex infection". Dermatologica. 156 (5): 257–267. doi:10.1159/000250926. PMID 640102.
- ^ Smriga and Torii; Torii, K (2003). "l-Lysine acts like a partial serotonin receptor 4 antagonist and inhibits serotonin-mediated intestinal pathologies and anxiety in rats". PNAS 100 (26): 15370–5. doi:10.1073/pnas.2436556100. PMC 307574. PMID 14676321. http://www.pnas.org/content/100/26/15370.short.
- ^ Smriga, Kameishi, Uneyama, and Torii (December 2002). "Dietary L-Lysine Deficiency Increases Stress-Induced Anxiety and Fecal Excretion in Rats". The Journal of Nutrition 132:3744-3746 (12): 3744–6. PMID 12468617. http://jn.nutrition.org/cgi/content/abstract/132/12/3744.
- ^ Smriga, Ghosh, Mouneimne, Pellett, and Scrimshaw (May 2004). "Lysine fortification reduces anxiety and lessens stress in family members in economically weak communities in Northwest Syria". Proceedings of the National Academy of Sciences 101 (22): 8285–8288. doi:10.1073/pnas.0402550101. http://www.pnas.org/content/101/22/8285.full.
- ^ ScienceDaily. "Chemists Kill Cancer Cells With Light-activated Molecules". http://www.sciencedaily.com/releases/2007/08/070808132019.htm. Retrieved 2008-01-24.
- ^ Chen C, Sander JE, Dale NM (2003). "The effect of dietary lysine deficiency on the immune response to Newcastle disease vaccination in chickens". Avian Dis. 47 (4): 1346–51. doi:10.1637/7008. PMID 14708981.
- ^ Flodin 1997[clarification needed]
- ^ a b "Norwegian granted for improving lysine production process"
- ^ Toride Y. "Lysine and other amino acids for feed: production and contribution to protein utilization in animal feeding". http://www.fao.org/docrep/007/y5019e/y5019e0a.htm. Retrieved 2011-01-25.
- ^ Coyne, Jerry A. (October 10, 1999). "The Truth Is Way Out There". The New York Times. http://query.nytimes.com/gst/fullpage.html?res=9E0DE6D7153EF933A25753C1A96F958260. Retrieved 2008-04-06.
- ^ Connor, J.M.; "Global Price Fixing" 2nd Ed. Springer-Verlag: Heidelberg, 2008. ISBN 978-3-540-78669-6.
- ^ Eichenwald, Kurt.; "The Informant: a true story" Broadway Books: New York, 2000. ISBN 0-7679-0326-9.
Sources
- Much of the information in this article has been translated from German Wikipedia.
- Lide, D. R., ed (2002). CRC Handbook of Chemistry and Physics (83rd ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0483-0.
The 20 common amino acids By properties AliphaticBranched-chain amino acids (Valine · Isoleucine · Leucine) · Methionine · Alanine · Proline · GlycineAromaticPolar, unchargedPositive charge (pKa)Negative charge (pKa)GeneralOther classifications biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iK→acetyl-CoA lysine→G G→pyruvate→citrateG→glutamate→
α-ketoglutarateotherα-Ketoisovaleric acid · Isobutyryl-CoA · Methacrylyl-CoA · 3-Hydroxyisobutyryl-CoA · 3-Hydroxyisobutyric acid · 2-Methyl-3-oxopropanoic acidG→fumarateG→oxaloacetatesee urea cycleOther biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Proteinogenic amino acids
- Ketogenic amino acids
- Basic amino acids
- Essential amino acids
Wikimedia Foundation. 2010.