- Disodium tetracarbonylferrate
-
Disodium tetracarbonylferrate 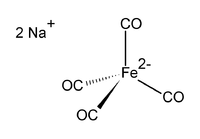 disodium tetracarbonylferrateSystematic namedisodium tetracarbonylferrateOther namesdisodium iron tetracarbonyl, Collman's reagent
disodium tetracarbonylferrateSystematic namedisodium tetracarbonylferrateOther namesdisodium iron tetracarbonyl, Collman's reagentIdentifiers CAS number 14878-31-0 Properties Molecular formula C4FeNa2O4 Molar mass 213.87 Appearance Colorless solid Density 2.16 g/cm3, solid Solubility in water Decomposes Solubility tetrahydrofuran, dimethylformamide, dioxane Structure Crystal structure Distorted tetrahedron Coordination
geometryTetrahedral Hazards Main hazards Pyrophoric Related compounds Related compounds Iron pentacarbonyl  tetracarbonylferrate (verify) (what is:
tetracarbonylferrate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Disodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe(CO)4]. This oxygen-sensitive colourless solid is employed in organic synthesis",[1] mainly to synthesise aldehydes.[2] It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's reagent.[3] The tetracarbonylferrate dianion is tetrahedral.[4]
Contents
Synthesis
The reagent was reported by Cooke in 1970.[5] The current synthesis entails the reduction of a solution of iron pentacarbonyl in tetrahydrofuran by sodium naphthenide. The efficiency of the synthesis depends on the quality of the iron pentacarbonyl.[1]
Reactions
The reagent was originally described for the conversion of primary alkyl bromides, RBr, to the corresponding aldehydes in a two-step, "one-pot" reaction:[5]
- Na2[Fe(CO)4] + RBr → Na[RFe(CO)4] + NaBr
This solution is then treated sequentially with PPh3 and then acetic acid to give the aldehyde, RCHO.
Disodium tetracarbonylferrate can be used to convert acid chlorides to aldehydes. As for Cooke’s early discovery, an iron acyl complex undergoes protonolysis to give the aldehyde.
- Na2[Fe(CO)4] + RCOCl → Na[RC(O)Fe(CO)4] + NaCl
- Na[RC(O)Fe(CO)4] + HCl → RCHO + "Fe(CO)4" + NaCl
Disodium tetracarbonylferrate reacts with alkyl halides (RX) to produce alkyl complexes:
- Na2[Fe(CO)4] + RX → Na[RFe(CO)4] + NaX
Such iron alkyls can be converted to the corresponding carboxylic acid and acid halides:
- Na[RFe(CO)4] + O2, H+ →→ RCO2H + Fe...
- Na[RFe(CO)4] + X2 → RC(O)X + FeX2 + 3 CO + NaCl
One attraction of these methods is the low cost of the iron carbonyl as well as the fact that the procedures are relatively “green” because the side product is iron-based.
References
- ^ a b Strong, H.; Krusic, P. J.; San Filippo, J. (1990). Robert J. Angelici. ed. "Sodium Carbonyl Ferrate, Na2Fe(CO)], Na2[Fe2(CO)8], and Na2[Fe3(CO)11]. Bis[μ-Nitrido-Bis(triphenylphosphorus)(1+] Undecarcarbonyltriferrate(2-), [(Ph3P)2N]2[Fe3(CO)11]". Inorganic Syntheses. Inorganic Syntheses (New York: J. Wiley & Sons) 28: 203–207. doi:10.1002/9780470132593.ch52. ISBN 0-471-52619-3.
- ^ Pike, Robert D., (2001). Disodium Tetracarbonylferrate (II-). Encyclopedia of Reagents for Organic Synthesis.
- ^ Miessler, G. L., Tarr, D. A. (2004). Inorganic chemistry. Upper Saddle River,New Jersey: Pearson Publication.
- ^ H. B. Chin, R. Bau (1976). "The Crystal Structure of Disodium Tetracarbonylferrate. Distortion of the Tetracarbonylferrate(2-) Anion in the Solid State". J. Am. Chem. Soc. 98 (9): 2434–2439. doi:10.1021/ja00425a009.
- ^ a b M. P. Cooke (1970). "Facile conversion of alkyl bromides into aldehydes using sodium tetracarbonylferrate(-II)". J. Am. Chem. Soc. 92 (20): 6080–6082. doi:10.1021/ja00723a056.
Further reading
- J. P. Collman (1975). "Disodium Tetracarbonylferrate, a Transition Metal Analog of a Grignard reagent". Accounts of Chemical Research 8 (10): 342–347. doi:10.1021/ar50094a004.
- C. Ungurenasu, C. Cotzur (1982). "Disodium Tetracarbonylferrate: A Reagent for Acid Functionalization of Halogenated Polymers". Journal Polymer Bulletin 6 (5–6): 299–303. doi:10.1007/BF00255401.
- V. W. Hieber, G. Braun (1959). Zeitschrift für Naturforschung 146: 132.
Categories:- Carbonyl complexes
- Iron compounds
Wikimedia Foundation. 2010.
