- Cyclobenzaprine
-
Cyclobenzaprine 

Systematic (IUPAC) name 3-(5H-dibenzo[a,d]cyclohepten-5-ylidene)- N,N-dimethyl-1-propanamine Clinical data Trade names Flexeril AHFS/Drugs.com monograph MedlinePlus a682514 Pregnancy cat. Category B Legal status ℞ Prescription only Routes PO (Per Oral) Pharmacokinetic data Bioavailability 33% to 55% [1][2] Metabolism hepatic Half-life 18 hours (range 8–37 hours; n=18) Excretion renal Identifiers CAS number 303-53-7 
ATC code M03BX08 PubChem CID 2895 DrugBank APRD00213 ChemSpider 2792 
UNII 69O5WQQ5TI 
KEGG D07758 
ChEBI CHEBI:3996 
ChEMBL CHEMBL669 
Chemical data Formula C20H21N Mol. mass 275.387 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Cyclobenzaprine is a muscle relaxant medication used to relieve skeletal muscle spasms and associated pain in acute musculoskeletal conditions.[3] It is the most well-studied drug for this application,[4] and it also has been used off-label for fibromyalgia treatment.[5]
Contents
Mechanism of action
The mechanism of action for cyclobenzaprine is unclear. Studies from the 1980s in rats indicate that cyclobenzaprine activates the locus ceruleus in the brain stem, leading to an increased release of norepinephrine in the ventral horn of the spinal cord and the subsequent inhibitory action of norepinephrine on alpha motor neurons.[6]
Cyclobenzaprine has been considered structurally related to the first-generation tricyclic antidepressants.[7] Such tricyclics, including amitriptyline, act to inhibit the uptake of norepinephrine, resulting in increased transynaptic norepinephrine concentration. They have been shown to exert analgesic effects in chronic nerve and muscle pain. Cyclobenzaprine may have a similar effect.
Others contend that the structure is more closely related to cyproheptadine,[8][9] an antagonist at histamine H1 receptors, muscarinic acetylcholine receptors, and 5-HT2A serotonin receptors. Corroborating studies show that cyclobenzaprine causes inhibition of descending serotonergic systems in the spinal cord by blocking 5-HT2A and 5-HT2C receptors.[10] This action is thought to have an inhibitory effect on the alpha motor neurons in the ventral horn of the spinal cord, thereby resulting in decreased firing of alpha-motor neurons and a reduction in spinal mono- and polysynaptic reflexes.[11]
Use
After sustaining an injury, muscle spasms may occur to stabilize the affected body part and prevent further damage. They also generate pain. Cyclobenzaprine is FDA-approved to treat such muscle spasm associated with acute, painful musculoskeletal conditions.[3] It decreases pain in the first two weeks,[4][12] peaking in the first few days, but has no proven benefit after two weeks.[4][13] Since no benefit is proven beyond that, therapy should not be continued long-term.[14] It is not useful for spasticity due to neurologic conditions such as cerebral palsy.[14][15]
Cyclobenzaprine has also shown effectiveness in the treatment of fibromyalgia symptoms, with a report of 4.8 patients needing treatment for each (1) patient reporting pain reduction (but no change in fatigue or tender points).[16] Like other tricyclic antidepressants, it is also prescribed off-label as a sleep-aid.[citation needed]
Formulations and dosages
Cyclobenzaprine is marketed as Apo-Cyclobenzaprine (10 mg tablets), Flexeril (5 and 10 mg tablets) and Fexmid (7.5 mg tablet). Both Flexeril and Fexmid are available in generic form. A once-a-day extended-release formulation Amrix is available in 15- and 30-mg capsules.[17]
Cyclobenzaprine is regulated in the U.S. for prescription use only. Though it does not fall within most governmental guidelines as a controlled substance, possession of it without a valid or current prescription may be illegal, depending upon various state and local laws.
Side effects
Meta-analysis studies have found significantly increased rates of drowsiness (38% of patients), dry mouth (24%), dizziness (10%), and adverse events of any kind in patients taking cyclobenzaprine versus placebo.[13] Drowsiness and dry mouth appear to intensify with increasing dose.[18]
Other side effects are not significantly more common than they are in patients taking placebo. Some of these include blurred vision, fatigue, nausea, and headache.[18] The sedative effects of cyclobenzaprine are likely due to its antagonistic effect on histamine, serotonin, and muscarinic receptors. Agitation is a common side effect observed especially in the elderly. In general, the NCQA recommends avoiding the use of cyclobenzaprine in the elderly because of the potential for more severe side effects.[19] There is one case report of overdose causing rhabdomyolysis (muscle breakdown).[20] Treatment protocols and support should follow the same as for any tricyclic antidepressant.[20]
Overdose
The most common effects of overdose are drowsiness and tachycardia.[3] Rare but potentially critical complications are cardiac arrest, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome.[3] Life-threatening overdose is rare,[3] however, as the median lethal dose is approximately 338 mg/kg in mice and 425 mg/kg in rats,[3]. The potential harm is increased when central nervous system depressants and antidepressants are also used; deliberate overdose often includes alcohol among other drugs.[3]
Interactions
Cyclobenzaprine has major contraindications with monoamine oxidase inhibitors (MAOIs). At least one study also found increased risk of serotonin syndrome when cyclobenzaprine was taken with the serotonergic drugs duloxetine or phenelzine.[21]
The following substances may interact with cyclobenzaprine:
- Alcohol
- Central nervous system (CNS) depressants (medicines that cause drowsiness)
- Tricyclic antidepressants may increase the chance of side-effects
- Monoamine oxidase inhibitors taken within 2 weeks of cyclobenzaprine may result in serious, life-threatening side-effects[14]
Chemistry
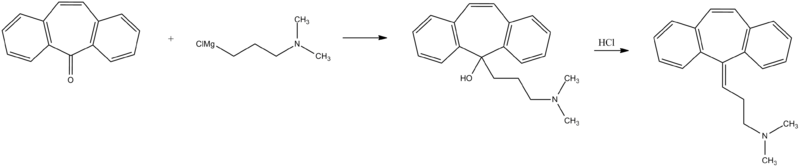
Cyclobenzaprine, N,N-dimethyl-3-(dibenzo[a,d]cyclohepten-5-ylidene)propylamine, is synthesized by reacting 5H-dibenzo[a,d]cyclohepten-5-one with 3-dimethylaminopropylmagnesium chloride and subsequent dehydration of the resulting carbinol in acidic conditions into cyclobenzaprine.
- H.La Roche, GB 858187 (1961).
- F.J. Villani, C.A. Ellis, C. Teihman, C. Biges, J. Med. Pharm. Chem., 5, 373 (1962).
- Winthrop, S. O.; Davis, M. A.; Myers, G. S.; Gavin, J. G.; Thomas, R.; Barber, R. (1962). "New Psychotropic Agents. Derivatives of Dibenzo[a,d]-1,4-cycloheptadiene". The Journal of Organic Chemistry 27: 230. doi:10.1021/jo01048a057.
Notes
- ^ Micromedex® 2010 - DRUGDEX® Evaluations (Cyclobenzaprine Hydrochloride)
- ^ Cyclobenzaprine HCl Oral tablets USP Revised, April 2005
- ^ a b c d e f g "Flexeril (Cyclobenzaprine HCl) Tablets" (pdf), U. S. Food and Drug Administration, 2003, http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/17821se8-045_flexeril_lbl.pdf, retrieved 26 July 2009
- ^ a b c Chou R, Peterson K, Helfand M (2004). "Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review". Journal of Pain Symptom Management 28 (2): 140–175. doi:10.1016/j.jpainsymman.2004.05.002. PMID 15276195.
- ^ "Cyclobenzaprine Hydrochloride" (subscription required). MicroMedex. 5 Feb 2010. http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/367361/DUPLICATIONSHIELDSYNC/A4E03B/ND_PG/PRIH/ND_B/HCS/SBK/1/ND_P/Main/PFActionId/hcs.common.RetrieveDocumentCommon/DocId/151425/ContentSetId/100/SearchTerm/flexeril/SearchOption/BeginWith. Retrieved 16 Feb 2010.
- ^ Commissiong JW; Karoum F; Reiffenstein RJ; Neff NH (Jan 1981). "Cyclobenzaprine: a possible mechanism of action for its muscle relaxant effect". Canadian Journal of Physiology and Pharmacology 59 (1): 37–44. PMID 7214207.
- ^ E.J. Preston, C.B. Miller and R.K. Herbertson, A double-blind, multicenter trial of methocarbamol (Robaxin) and cyclobenzaprine (Flexeril(TM)) in acute musculoskeletal conditions. Today's Ther Trends 1 4 (1984), pp. 1–11.
- ^ Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al (2006). "DrugBank: a comprehensive resource for in silico drug discovery and exploration". Nucleic Acids Ressearch 34 (Database issue): D668–D672.
- ^ Gillman PK (May 2009). "Is there sufficient evidence to suggest cyclobenzaprine might be implicated in causing serotonin toxicity?" (comment letter). The American Journal Of Emergency Medicine 27 (4): 509–10; author reply 510. PMID 19555629.
- ^ Honda M, Nishida T & Ono H (2003). "Tricyclic analogs cyclobenzaprine, amitriptyline, and cyproheptadine inhibit the spinal reflex transmission through 5-HT(2) receptors". European Journal of Pharmacology 458: 919. PMID 12498911.
- ^ Kobayashi H, Hasegawa Y & Ono H (1996). "Cyclobenzaprine, a centrally acting muscle relaxant, acts on descending serotonergic systems". European Journal of Pharmacology 311: 29–35. doi:10.1016/0014-2999(96)00402-5. PMID 8884233.
- ^ van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM (2003). "Muscle relaxants for non-specific low back pain". Cochrane Database Systematic Reviews 2: CD004252. PMID 12498911.
- ^ a b Browning R, Jackson JL, O’Malley PG (2001). "Cyclobenzaprine and back pain: a meta-analysis". Archives of Internal Medicine 161 (13): 1613–1620. PMID 11434793.
- ^ a b c "Cyclobenzaprine official FDA information, side effects, and uses". Drugs.com. October 2009. http://www.drugs.com/pro/cyclobenzaprine.html. Retrieved 19 Feb 2010.
- ^ Ashby P, Burke D, Rao S, et al (1972). "Assessment of cyclobenzaprine in the treatment of spasticity". J Neurol Neurosurg Psychiatry 35 (5): 599–605. doi:10.1136/jnnp.35.5.599. PMC 494138. PMID 4563483. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=494138.
- ^ Tofferi JK, Jackson JL, O'Malley PG (15 Feb 2004). "Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis". Arthritis & Rheumatology 51 (1): 9–13. PMID 14872449.
- ^ Amrix website
- ^ a b "Flexeril: Side effects". RxList.com. http://www.rxlist.com/cgi/generic/cyclobnz_ad.htm. Retrieved 22 Feb 2010.
- ^ "High risk medications" (pdf). National Committee for Quality Assurance. http://www.ncqa.org/Portals/0/Newsroom/SOHC/Drugs_Avoided_Elderly.pdf. Retrieved 22 Feb 2010.
- ^ a b Chabria, Shiven B (17 July 2006). "Rhabdomyolysis: a manifestation of cyclobenzaprine toxicity". Journal of Occupational Medicine and Toxicology 1 (1): 16. doi:10.1186/1745-6673-1-16. PMC 1540431. PMID 16846511. http://www.occup-med.com/content/1/1/16.
- ^ Keegan MT, Brown DR & Rabinstein AA (2006). "Serotonin syndrome from the interaction of cyclobenzaprine with other serotoninergic drugs". Anesthesia & Analgesia 103: 14668. PMID 17122225.
Articles and topics related to Cyclobenzaprine Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersTricyclics Classes Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • ThioxantheneAntidepressants 7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • TrimipramineAntihistamines Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • ThiazinamiumAntipsychotics Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • ZuclopenthixolOthers Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • ProfenamineSkeletal muscle relaxants (M03) Peripherally acting
(primarily antinicotinic,
NMJ block)Curare alkaloidsultra-short duration: Gantacurium
short duration: Mivacurium • Chandonium
intermediate duration: Atracurium • Cisatracurium • Fazadinium • Rocuronium • Vecuronium
long duration: Doxacurium • Dimethyltubocurarine • Pancuronium • Pipecuronium • Laudexium • Gallamine
unsorted: Hexafluronium (Hexafluorenium)Choline derivatives: Suxamethonium (Succinylcholine)
Polyalkylene derivatives: HexamethoniumCentrally acting Carbamic acid estersBenzodiazepinesAnticholinergics (Antimuscarinics)Cyclobenzaprine • OrphenadrineOtherBaclofen • Chlormezanone • Chlorphenesin • Chlorzoxazone • Donepezil • Eperisone • Flopropione • Mephenesin • Mephenoxalone • Metaxalone • Phenyramidol • Pridinol • Promoxolane • Quinine • Thiocolchicoside • Tizanidine • Tolperisone • TrazodoneDirectly acting Categories:- Muscle relaxants
Wikimedia Foundation. 2010.
Look at other dictionaries:
cyclobenzaprine — noun A particular antidepressant generally prescribed as an analgesic and muscle relaxant … Wiktionary
cyclobenzaprine — cy·clo·ben·za·prine ben zə .prēn, prən n a skeletal muscle relaxant administered in the form of its hydrochloride C20H21N·HCl to relieve muscle spasms and pain … Medical dictionary
cyclobenzaprine — noun muscle relaxant (trade name Flexeril) used for muscle spasms or acute injury • Syn: ↑Flexeril • Usage Domain: ↑trade name (for: ↑Flexeril) • Hypernyms: ↑muscle relaxant … Useful english dictionary
cyclobenzaprine hydrochloride — A centrally acting skeletal muscle relaxant used to relieve acute muscular spasms. * * * cy·clo·ben·za·prine hy·dro·chlo·ride (si″klo benґzə prēn) [USP] a compound structurally related to the tricyclic antidepressants, used as a… … Medical dictionary
Muscle relaxant — This article refers to skeletal muscle relaxants. For information on smooth muscle relaxants, see Antispasmodic. A muscle relaxant is a drug which affects skeletal muscle function and decreases the muscle tone. It may be used to alleviate… … Wikipedia
Orphenadrine — Systematic (IUPAC) name N,N dimethyl 2 [(2 methylphenyl) phenyl methoxy] ethanamine Clinical data Trade names … Wikipedia
Methorphan — Systematic (IUPAC) name 3 methoxy 17 methylmorphinan Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number … Wikipedia
Methadone — Phy redirects here. For the abbreviation for the physical layer of the OSI Model, see PHY. Not to be confused with Methedrine, Methedrone, Mephedrone, or Methylone. Methadone … Wikipedia
Phencyclidine — Systematic (IUPAC) name … Wikipedia
Clozapine — Not to be confused with clonazepam or Klonopin. Clozapine Systematic (IUPAC) name … Wikipedia

