- Convective heat transfer
-
See also: Heat transfer and convection
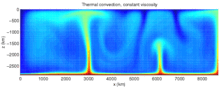 This figure shows a calculation for thermal convection. Colors closer to red are hot areas and colors closer to blue are cold areas. In this figure, a hot, less-dense lower boundary layer sends plumes of hot material upwards, and likewise, cold material from the top moves downwards. This figure is from a model of convection in the Earth's mantle.
This figure shows a calculation for thermal convection. Colors closer to red are hot areas and colors closer to blue are cold areas. In this figure, a hot, less-dense lower boundary layer sends plumes of hot material upwards, and likewise, cold material from the top moves downwards. This figure is from a model of convection in the Earth's mantle.
Convective heat transfer, often referred to as convection, is the transfer of heat from one place to another by the movement of fluids. The presence of bulk motion of the fluid enhances the heat transfer between the solid surface and the fluid.[1] Convection is usually the dominant form of heat transfer in liquids and gases. Although often discussed as a third method of heat transfer, convective heat transfer actually describes the combined effects of conduction (heat diffusion), plus heat transfer by bulk fluid flow, a process technically termed "heat advection."
The term convection can refer to transfer of heat with any fluid movement, but advection is the more precise term for the transfer due only to bulk fluid flow. The process of transfer of heat from a solid to a fluid, or the reverse, requires not only transfer of heat by bulk motion of the fluid, but also diffusion/conduction of heat through the still boundary layer next to the solid. Thus, this process with a moving fluid requires both diffusion and advection of heat, a summed process that is generally called convection.
Convection can be "forced" by movement of a fluid by means other than buoyancy forces (for example, a water pump in an automobile engine). In some cases, natural buoyancy forces alone are entirely responsible for fluid motion when the fluid is heated, and this process is called "natural convection." An example is the draft in a chimney or around any fire. In natural convection, an increase in temperature produces a reduction in density, which causes fluid motion due to pressures and forces when fluids of different densities are affected by gravity (or any g-force). For example, when water is heated on a stove, hot water from the bottom of the pan rises, displacing the colder denser liquid which falls. After heating has stopped, mixing and conduction from this natural convection eventually result in a nearly homogeneous density, and even temperature.
The convection heat transfer mode is comprised to two mechanisms. In addition to energy transfer due to random molecular motion (diffusion), energy is also transferred by bulk, or macroscopic, motion of the fluid. This motion is associated with the fact that, at any instant, large numbers of molecules are moving collectively or as aggregates. Such motion, in the presence of a temperature gradient, contributes to heat transfer. Because the molecules in aggregate retain their random motion, the total heat transfer is then due to the superposition of energy transport by random motion of the molecules and by the bulk motion of the fluid. It is customary to use the term convection when referring to this cumulative transport and the term advection when referring to the transport due to bulk fluid motion.[2]
Contents
Overview
Convection is the transfer of heat from one place to another by the movement of fluids. The presence of bulk motion of the fluid enhances the heat transfer between the solid surface and the fluid.[3] Convection is usually the dominant form of heat transfer in liquids and gases. Although often discussed as a third method of heat transfer, convection actually describes the combined effects of conduction and fluid flow.
There are two types of convective heat transfer
- Free or natural convection: when the fluid motion is caused by buoyancy forces that result from the density variations due to variations of temperature in the fluid. In the absence of an external source, when the mass of the fluid is in contact with a hot surface, its molecules separate and scatter, causing the mass of fluid to become less dense. When this happens, the fluid is displaced vertically or horizontally while the cooler fluid gets denser and the fluid sinks. Thus the hotter volume transfers heat towards the cooler volume of that fluid.[4] Familiar examples are the upward flow of air due to a fire or hot object and the circulation of water in a pot that is heated from below.
- Forced convection: when the fluid is forced to flow over the surface by external source such as fans, stirrers, and pumps, creating an artificially induced convection current.[5]
Internal and external flow can also classify convection. Internal flow occurs when the fluid is enclosed by a solid boundary such as a flow through a pipe. An external flow occurs when the fluid extends indefinitely without encountering a solid surface. Both of these convections, either natural or forced, can be internal or external because they are independent of each other.[citation needed] The bulk temperature, or the average fluid temperature, is a convenient reference point for evaluating properties related to convective heat transfer, particularly in applications related to flow in pipes and ducts.
For a visual experience of natural convection, a glass that is full of hot water filled with red food dye may be placed inside a fish tank with cold, clear water. The convection currents of the red liquid will be seen to rise and also fall, then eventually settle, illustrating the process as heat gradients are dissipated.
Equations (Newton's law of cooling)
Convection-cooling can sometimes be described by Newton's law of cooling in cases where the heat transfer coefficient is independent or relatively independent of the temperature difference between object and environment. This is sometimes true, but is not guaranteed to be the case (see other situations below where the transfer coefficient is temperature dependent).
Newton's law, which requires a constant heat transfer coefficient, states that the rate of heat loss of a body is proportional to the difference in temperatures between the body and its surroundings. The rate of heat transfer in such circumstances is derived below:[6]
Newton's cooling law is a solution of the differential equation given by Fourier's law:
where
- Q is the thermal energy in joules
- h is the Heat transfer coefficient (assumed independent of T here)
- A is the surface area of the heat being transferred
- T is the temperature of the object's surface and interior (since these are the same in this approximation)
- Tenv is the temperature of the environment; i.e. the temperature suitably far from the surface
- ΔT(t) = T(t) − Tenv is the time-dependent thermal gradient between environment and object
The heat transfer coefficient h depends upon physical properties of the fluid and the physical situation in which convection occurs. Therefore, a single usable heat transfer coefficient (one that does not vary significantly across the temperature-difference ranges covered during cooling and heating) must be derived or found experimentally for every system analyzed. Formulas and correlations are available in many references to calculate heat transfer coefficients for typical configurations and fluids. For laminar flows, the heat transfer coefficient is rather low compared to the turbulent flows; this is due to turbulent flows having a thinner stagnant fluid film layer on heat transfer surface.[7] However, note that Newton's law breaks down if the flows should transition between laminar or turbulent flow, since this will change the heat transfer coefficient h which is assumed constant in solving the equation.
Newton's law requires that internal heat conduction within the object be large in comparison to the loss/gain of heat by convection (lumped capacitance model), and this may not be true (see heat transfer). Also, an accurate formulation for temperatures may require analysis based on changing heat transfer coefficients at different temperatures, a situation frequently found in free-convection situations, and which precludes accurate use of Newton's law. Assuming these are not problems, then the solution can be given if heat transfer within the object is considered to be far more rapid than heat transfer at the boundary (so that there are small thermal gradients within the object). This condition, in turn, allows the heat in the object to be expressed as a simple product of the object's mass, its heat capacity, and its temperature, as in the following section:
Solution in terms of object heat capacity
If the entire body is treated as lumped capacitance thermal energy reservoir, with a total thermal energy content which is proportional to simple total heat capacity C, and T, the temperature of the body, or Q = CT. It is expected that the system will experience exponential decay with time in the temperature of a body.
From the definition of heat capacity C comes the relation C = dQ / dT. Differentiating this equation with regard to time gives the identity (valid so long as temperatures in the object are uniform at any given time): dQ / dt = C(dT / dt). This expression may be used to replace dQ / dt in the first equation which begins this section, above. Then, if T(t) is the temperature of such a body at time t, and Tenv is the temperature of the environment around the body:
where
r = hA / C is a positive constant characteristic of the system, which must be in units of s − 1, and is therefore sometimes expressed in terms of a characteristic time constant t0 given by: r = 1 / t0 = ΔT / (dT(t) / dt). Thus, in thermal systems, t0 = C / hA. (The total heat capacity C of a system may be further represented by its mass-specific heat capacity cp multiplied by its mass m, so that the time constant t0 is also given by mcp / hA).
The solution of this differential equation, by standard methods of integration and substitution of boundary conditions, gives:
If:
 is defined as :
is defined as :  where
where  is the initial temperature difference at time 0,
is the initial temperature difference at time 0,
then the Newtonian solution is written as:
This same solution is more immediately apparent if the initial differential equation is written in terms of ΔT(t), as a single function of time to be found, or "solved for." '
References
- ^ Yugnus A Cengel (2003), “Heat transfer-A Practical Approach” 2nd ed. Publisher McGraw Hill Professional, p26 by ISBN 0072458933, 9780072458930, Google Book Search. Accessed 20-04.-09
- ^ Incropera DeWitt VBergham Lavine 2007, Introduction to Heat Transfer, pg. 6 ISBN-13 978-471-45727-5
- ^ Yugnus A Cengel (2003), “Heat transfer-A Practical Approach” 2nd ed. Publisher McGraw Hill Professional, p26 by ISBN 0072458933, 9780072458930, Google Book Search. Accessed 20-04.-09
- ^ http://biocab.org/Heat_Transfer.html Biology Cabinet organization, April 2006, “Heat Transfer”, Accessed 20/04/09
- ^ http://www.engineersedge.com/heat_transfer/convection.htm Engineers Edge, 2009, “Convection Heat Transfer”,Accessed 20/04/09
- ^ Louis C. Burmeister, (1993) “Convective Heat Transfer”, 2nd ed. Publisher Wiley-Interscience, p 107 ISBN 047157709X, 9780471577096, Google Book Search. Accessed 20-03-09
- ^ http://www.engineersedge.com/heat_transfer/convection.htm Engineers Edge, 2009, “Convection Heat Transfer”,Accessed 20/03/09
See also
- Heat transfer coefficient
- Heisler Chart
- Thermal conductivity
Categories:
Wikimedia Foundation. 2010.






