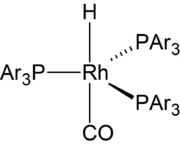- Hydroformylation
-
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes.[1] This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.
The process typically is accomplished by treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.
Contents
Catalysts
The original catalyst was HCo(CO)4, discovered by Otto Roelen.[2][3] Subsequent work demonstrated that the ligand tributylphosphine (PBu3) improved the selectivity of the cobalt-catalysed process. Since the 1970s, most hydroformylation relies on catalysts based on rhodium.[4][5] Subsequent research led to the development of water-soluble catalysts that facilitate the separation of the products from the catalyst.[6]
Mechanism
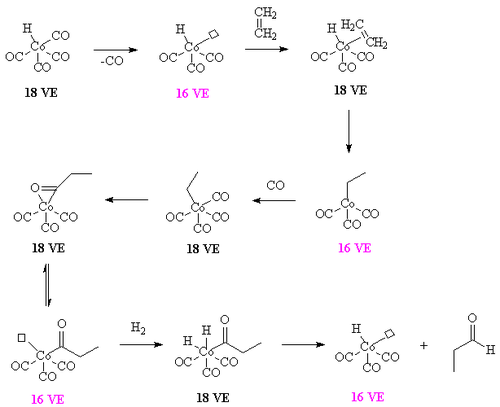
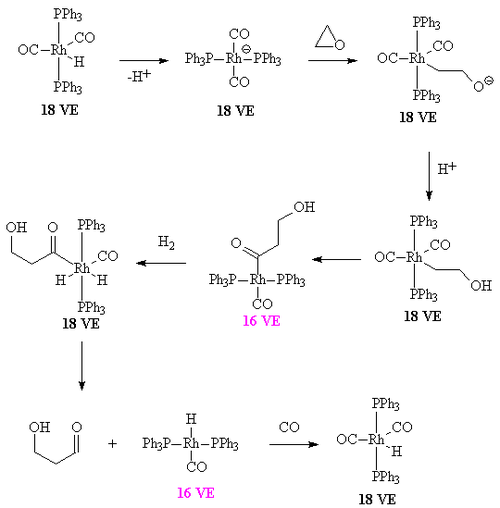
The overall mechanism resembles that for homogeneous hydrogenation with additional steps. The reaction begins with the generation of coordinatively unsaturated metal hydrido carbonyl complex such as HCo(CO)3 and HRh(CO)(PPh3)2. Such species bind alkenes, and the resulting complex undergoes a migratory insertion reaction to form an alkyl complex.
Selectivity
A key consideration of hydroformylation is the "normal" vs. "iso" selectivity. For example, the hydroformylation of propylene can afford two isomeric products, butyraldehyde or isobutyraldehyde:
- H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO ("normal")
- vs.
- H2 + CO + CH3CH=CH2 → (CH3)2CHCHO ("iso")
These isomers result from the differing ways of inserting the alkene into the M-H bond. Of course, both products are not equally desirable. Much research was dedicated to the quest for catalyst that favored the normal isomer.
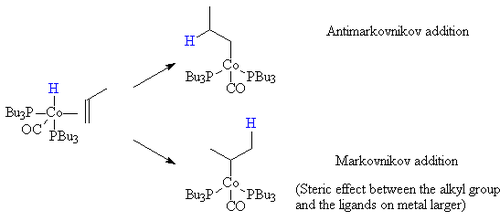
Steric effects
When the hydrogen is transferred to the carbon bearing the most hydrogen atoms (Markovnikov addition) the resulting alkyl group has a larger steric bulk close to the ligands on the cobalt. If the ligands on the cobalt are bulky (such as tributyl phosphine), then this steric effect is greater. Hence, the mixed carbonyl/phosphine complexes offer a greater selectivity toward the straight chain products.
Electronic effects
In addition, the more electron-rich the hydride complex is the less proton-like the hydride is. Thus, as a result, the electronic effects that favour the Markovnikov addition to an alkene are less able to direct the hydride to the carbon atom bearing the most hydrogens already. Thus, as a result, as the metal centre becomes more electron-rich, the catalyst becomes more selective for the straight chain compounds.
Acetyl formation
After the alkyl formation a second migatory insertion converts the alkyl into an acetyl ligand (this is when the alkyl carbon forms a bond with the carbon of a carbonyl ligand). The vacant site on the metal is filled by two hydrogens (from the oxidative insertion of a hydrogen molecule. One of these hydrides then takes part in a reductive elimination to form the molecule of the aldehyde and the complex [HCo(CO)3].
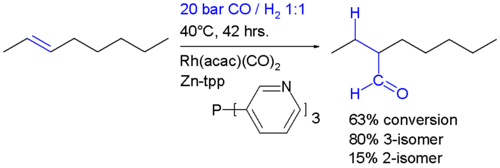
It is important that the rate of migatory insertion of the carbonyl into the carbon-metal bond of the alkyl is fast; in systems where the migatory insertion does not occur (such as nickel hydride tristriphenyl phosphite), the reaction of the hydride with the alkene is reversible. This results in the isomerisation of the alkene, in this way oct-2-ene could be converted into a mixture of both oct-1-ene and oct-2-ene by a beta hydride elimination from the alkyl. In the system below, the rate of insertion of the carbonyl carbon into the C-M bond is likely to be greater than the rate of beta-hydride elimination. If the converse was true then some n-C8H17CHO would have been formed.[7] Hydroformylation of 2-octene: the rhodium catalyst is coordinated to acac and carbon monoxide and encapsulated in a molecular self-assembly process by zinc tetraphenylporphyrin or Zn-tpp and the pyridine analogue of triphenylphosphine. In this process very much like the way enzymes work encapsulation of the catalytic site explains the observed regioselectivity:
Asymmetric hydroformylation
Hydroformylation of internal alkenes creates new stereocenters. Using chiral phosphine ligands, the hydroformylation can be tailored to favor one enantiomer.[8]
Other substrates
Cobalt carbonyl and rhodium complexes catalyse the hydroformylation of formaldehyde and ethylene oxide to give 2-hydroxyacetaldehyde and 3-hydroxypropanaldehyde, which can then be hydrogenated to ethylene glycol and 1,3-propanediol, respectively. The reactions work best when the solvent is basic (such as pyridine).[9][10]
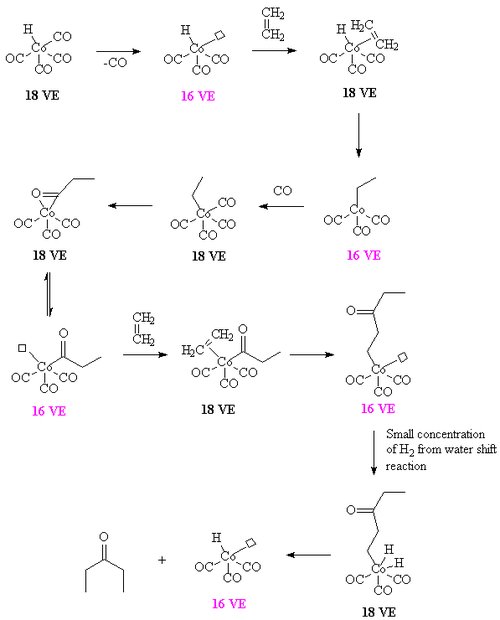
In the case of dicobalt octacarbonyl or Co2(CO)8 as a catalyst, 2-pentanone can arise from ethylene and CO, in the absence of hydrogen. A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes a migratory insertion to form [CH3COCH2CH2Co(CO)3]. The required hydrogen arises from the water shift reaction. For details, see [11]
If the water shift reaction is not operative, the reaction affords a polymer containing alternating carbon monoxide and ethylene units. Such aliphatic polyketones are more conventionally prepared using palladium catalysts.[12]
References
- ^ Ojima, I.; Tsai, C.-Y.; Tzamarioudaki, M.; Bonafoux, D. Org. React. 2000, 56, 1. (doi: 10.1002/0471264180.or056.01)
- ^ Boy Cornils, Wolfgang A. Herrmann, Manfred Rasch (1994). "Otto Roelen, Pioneer in Industrial Homogeneous Catalysis". Angewandte Chemie International Edition in English 33 (21): 2144–2163. doi:10.1002/anie.199421441.
- ^ http://www.tu-braunschweig.de/Medien-DB/anchem/homogenekat-4bw.pdf
- ^ Imyanitov N.S., Rudkovskij D.M. (1963). "Hydrogenation and hydroformylation in the presence of Co, Rh, and Ir carbonyls". Neftekhimiya (Rus) 3 (2): 198–200.
- ^ Evans D., Osborn J. A., Wilkinson G. (1968). "Hydroformylation of Alkenes by Use of Rhodium Complex Catalyst". Journal of the Chemical Society 33 (21): 3133–3142. doi:10.1039/J19680003133. http://www.rsc.org/ejarchive/J1/1968/J19680003133.pdf.
- ^ Cornils, B.; Herrmann, W. A. (eds.) “Aqueous-Phase Organometallic Catalysis” VCH, Weinheim: 1998
- ^ High-Precision Catalysts: Regioselective Hydroformylation of Internal Alkenes by Encapsulated Rhodium Complexes Kuil, M.; Soltner, T.; van Leeuwen, P. W. N. M.; Reek, J. N. H. Journal of the American Chemical Society; 2006; 128(35) pp 11344 - 11345; doi:10.1021/ja063294i
- ^ Watkins, A. L.; Hashiguchi, B. G.; Landis, C. R., Highly Enantioselective Hydroformylation of Aryl Alkenes with Diazaphospholane Ligands. Organic Letters 2008, 10 (20), 4553-4556.
- ^ Chan A.S.C., Shieh H-S. (1994). "A mechanistic study of the homogeneous catalytic hydroformylation of formaldehyde: synthesis and characterization of model intermediates". Inorganica Chimica Acta 218 (1–2): 89–95. doi:10.1016/0020-1693(94)03800-7.
- ^ Spencer A. (1980). "Hydroformylation of formaldehyde catalysed by rhodium complexes". Journal of Organometallic Chemistry 194 (1–2): 113–123. doi:10.1016/S0022-328X(00)90343-7.
- ^ Murata K., Matsuda A., (1981). "Application of Homogeneous Water-Gas Shift Reaction III Further Study of the Hydrocarbonylation - A highly Selective Formation of Diethyl Keton from Ethene, CO and H2O". Bulletin of the Chemical Society Japan 54 (7): 2089–2092. doi:10.1246/bcsj.54.2089.
- ^ J. Liu, B.T. Heaton, J.A. Iggo and R. Whyman (2004). "The Complete Delineation of the Initiation, Propagation, and Termination Steps of the Carbomethoxy Cycle for the Carboalkoxylation of Ethene by Pd–Diphosphane Catalysts". Angew. Chem. Int. Ed. 43: 90–94. doi:10.1002/anie.200352369.
Further reading
- “Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Two Volumes (Paperback) by Boy Cornils (Editor), W. A. Herrmann (Editor). ISBN 3-527-29594-1
- “Rhodium Catalyzed Hydroformylation” P. W. N. M. van Leeuwen, C. Claver Eds.; Springer; (2002). ISBN 1-4020-0421-4
- “Homogeneous Catalysis: Understanding the Art” by Piet W. N. M. van Leeuwen Springer; ISBN 2005. ISBN 1-4020-3176-9
- Imyanitov N.S./ Hydroformylation of Olefins with Rhodium Complexes // Rhodium Express. 1995. No 10 - 11 (May). P. 3 - 62 (Eng). ISSN 0869 - 7876
Categories:- Addition reactions
- Organometallic chemistry
- Homogeneous catalysis
Wikimedia Foundation. 2010.