- Chlorine trifluoride
-
Chlorine trifluoride 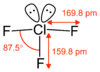
 Systematic nameTrifluoro-λ3-chlorane[1] (substitutive)
Systematic nameTrifluoro-λ3-chlorane[1] (substitutive)Identifiers CAS number 7790-91-2 
PubChem 24637 ChemSpider 23039 
EC number 232-230-4 UN number 1749 MeSH chlorine+trifluoride ChEBI CHEBI:30123 
RTECS number FO2800000 Gmelin Reference 1439 Jmol-3D images Image 1
Image 2- F[Cl](F)F
[F-].[F-].F[Cl++]
Properties Molecular formula ClF3 Molar mass 92.45 g mol−1 Exact mass 91.964062322 g mol-1 Appearance Colourless gas Density 4 mg cm-3 Melting point −76 °C, 197 K, -105 °F
Boiling point 12 °C, 285 K, 54 °F
Solubility in water Reacts Vapor pressure 175 kPa Viscosity 91.82 μPa s Structure Molecular shape T-shaped Thermochemistry Std enthalpy of
formation ΔfHo298−158.87 kJ mol-1[2] Standard molar
entropy So298281.59 J K−1mol−1[2] Hazards MSDS natlex.ilo.ch GHS pictograms 



GHS signal word DANGER EU classification  O
OR-phrases R8, R35 S-phrases S17, S38 NFPA 704 Related compounds Related compounds Chlorine pentafluoride
 trifluoride (verify) (what is:
trifluoride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colourless, poisonous, corrosive and very reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). The compound is primarily of interest as a component in rocket fuels, in industrial cleaning and etching operations in the semiconductor industry,[3][4] in nuclear reactor fuel processing,[5] and other industrial operations.[6]
Contents
Preparation, structure, and properties
Main article: chlorine fluoridesIt was first reported in 1930 by Ruff and Krug who prepared it by fluorination of chlorine; this also produced ClF and the mixture was separated by distillation.[7]
- 3 F2 + Cl2 → 2 ClF3
ClF3 is approximately T-shaped, with one short bond (1.598 Å) and two long bonds (1.698 Å).[8] This structure agrees with the prediction of VSEPR theory, which predicts lone pairs of electrons as occupying two equatorial positions of a hypothetic trigonal bipyramid. The elongated Cl-Faxial bonds are consistent with hypervalent bonding.
Pure ClF3 is stable to 180° in glass vessels; above this temperature it decomposes by a free radical mechanism to the elements.
The main use of ClF3 is to produce uranium hexafluoride, UF6, as part of nuclear fuel processing and reprocessing, by the reaction:
- U + 3 ClF3 → UF6 + 3 ClF
Hazards
ClF3 is a very strong oxidizing and fluorination agent. It is extremely reactive with most inorganic and organic materials, even plastics, and will initiate the combustion of many materials without an ignition source. These reactions are often violent, and in some cases explosive. Reaction with several metals give chlorides and fluorides; phosphorus yields PCl3 and PF5; and sulfur yields SCl2 and SF4. ClF3 is also violently reactive with water, in which it hydrolyses to a variety of hazardous chemicals such as hydrofluoric acid. H2S explodes on being mixed with ClF3 at room temperature.
The ability to surpass the oxidizing ability of oxygen leads to corrosivity against oxide-containing materials often thought as incombustible. In an industrial accident, a spill of 900 kg of chlorine trifluoride burned itself through 30 cm of concrete and 90 cm of gravel beneath.[9] Any equipment that comes into contact with chlorine trifluoride must be carefully checked and cleaned, because any contamination can ignite on contact. In addition, most general-purpose fire control/suppression hardware (Class A/B/C/K) is either incapable of suppressing this oxidation or can aggravate it; chlorine trifluoride has been reported to burn sand, asbestos, and other highly fire-retardant materials, reacts violently with water-based suppressors, and oxidizes in the absence of atmospheric oxygen, rendering atmosphere-displacement suppressors such as Halon and CO2 ineffective. It ignites glass on prolonged contact.[10]
Exposure of larger amounts of chlorine trifluoride, as a liquid or as a gas, ignites tissue. The hydrolysis reaction with water is violent and exposure results in a thermal burn. The product of hydrolysis is mainly hydrofluoric acid and hydrochloric acid, usually released as steam or vapor due to the highly exothermic nature of the reaction. Hydrofluoric acid is corrosive to human tissue, absorbs through skin, selectively attacks bone and stimulates pain nerves, and causes a potentially lethal poisoning. Hydrochloric acid is secondary in its danger to living organisms, but is more corrosive to most inorganic materials than hydrofluoric acid.
Military applications
Under the code name N-stoff ("substance N"), chlorine trifluoride was investigated for military applications by the Kaiser Wilhelm Institute in Nazi Germany from slightly before the start of World War II. Tests were made against mock-ups of the Maginot Line fortifications, and it was found to be an effective combined incendiary weapon and poison gas. From 1938 construction commenced on a partly bunkered, partly subterranean 31.76 km² munitions factory at Falkenhagen which was intended to produce 50 tonnes of N-stoff per month, plus Sarin. However, by the time it was captured by the advancing Red Army in 1944, the factory had produced only about 30 to 50 tonnes, at a cost of over 100 German Reichsmark per kilograma. N-stoff was never used in war.[11]
Rocket propellant
Chlorine trifluoride has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, prevented its use. John D. Clark summarized the difficulties:
It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water — with which it reacts explosively. It can be kept in some of the ordinary structural metals — steel, copper, aluminium, etc. — because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes."[12][13][14]
Semiconductor industry
In the semiconductor industry, chlorine trifluoride is used to clean chemical vapour deposition chambers.[15] It has the advantage that it can be used to remove semiconductor material from the chamber walls without having to dismantle the chamber.[15] Unlike most of the alternative chemicals used in this role, it does not need to be activated by the use of plasma since the heat of the chamber is enough to make it decompose and react with the semiconductor material.[15]
References
- ^ "chlorine trifluoride - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=24637. Retrieved 9 October 2011.
- ^ a b "chlorine trifluoride". NIST Chemistry WebBook. USA: National Institute of Standards and Technology. Gas phase thermochemistry data. http://webbook.nist.gov/cgi/cbook.cgi?ID=7790-91-2&Units=SI&cMS=on. Retrieved 9 October 2011.
- ^ Hitoshi Habuka, Takahiro Sukenobu, Hideyuki Koda, Takashi Takeuchi, and Masahiko Aihara (2004). "Silicon Etch Rate Using Chlorine Trifluoride". Journal of the Electrochemical Society 151 (11): G783–G787. doi:10.1149/1.1806391.
- ^ United States Patent 5849092 "Process for chlorine trifluoride chamber cleaning"
- ^ Board on Environmental Studies and Toxicology, (BEST) (2006). Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 5 (citation at the National Academies Press). Washington D.C.: National Academies Press. p. 40. ISBN 0-309-10358-4.
- ^ United States Patent 6034016 "Method for regenerating halogenated Lewis acid catalysts"
- ^ Otto Ruff, H. Krug (1930). "Über ein neues Chlorfluorid-CIF3". Zeitschrift für anorganische und allgemeine Chemie 190 (1): 270–276. doi:10.1002/zaac.19301900127.
- ^ Smith, D. F. (1953). "The Microwave Spectrum and Structure of Chlorine Trifluoride". The Journal of Chemical Physics 21 (4): 609–614. Bibcode 1953JChPh..21..609S. doi:10.1063/1.1698976.
- ^ Air Products Safetygram.http://web.archive.org/web/20060318221608/http://www.airproducts.com/nr/rdonlyres/8479ed55-2170-4651-a3d4-223b2957a9f3/0/safetygram39.pdf
- ^ Pradyot Patnaik (2007). A comprehensive guide to the hazardous properties of chemical substances (3rd ed.). Wiley-Interscience. p. 478. ISBN 0471714585.
- ^ "Bunker Tours" report on Falkenhagen
- ^ Clark, John D. (2001). Ignition!. UMI Books on Demand. ISBN 0-8135-0725-1.
- ^ ClF3/Hydrazine at the Encyclopedia Astronautica.
- ^ Clark, John D. (1972). Ignition! An Informal History of Liquid Rocket Propellants. Rutgers University Press. pp. 214. ISBN 0813507251.
- ^ a b c "In Situ Cleaning of CVD Chambers". Semiconductor International. 6/1/1999. http://www.semiconductor.net/article/209105-In_Situ_Cleaning_of_CVD_Chambers.php.
- Notes
- Groehler, Olaf (1989). Der lautlose Tod. Einsatz und Entwicklung deutscher Giftgase von 1914 bis 1945. Reinbek bei Hamburg: Rowohlt. ISBN 3-499-18738-8.
- Ebbinghaus, Angelika (1999). Krieg und Wirtschaft: Studien zur deutschen Wirtschaftsgeschichte 1939–1945. Berlin: Metropol. pp. 171–194. ISBN 3-932482-11-5.
- Harold Simmons Booth, John Turner Pinkston, , Jr. (1947). "The Halogen Fluorides". Chemical Reviews 41 (3): 421–439. doi:10.1021/cr60130a001.
- Yu D Shishkov, A A Opalovskii (1960). "Physicochemical Properties of Chlorine Trifluoride". Russian Chemical Reviews 29 (6): 357–364. doi:10.1070/RC1960v029n06ABEH001237.
- Robinson D. Burbank, Frank N. Bensey (1953). "The Structures of the Interhalogen Compounds. I. Chlorine Trifluoride at -120 °C". The Journal of Chemical Physics 21 (4): 602–608. doi:10.1063/1.1698975.
- A. A. Banks and A. J. Rudge (1950). "The determination of the liquid density of chlorine trifluoride". Journal of the Chemical Society: 191–193. doi:10.1039/JR9500000191.
- Lowdermilk, F. R.; Danehower, R. G.; Miller, H. C. (1951). "Pilot plant study of fluorine and its derivatives". Journal of Chemical Education 28 (5): 246. doi:10.1021/ed028p246.
^a Using data from Economic History Services and The Inflation Calculator, we can calculate that 100 Reichsmark in 1941 is approximately equivalent to $540 US dollars in 2006. Reichsmark exchange rate values from 1942 to 1944 are fragmentary.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- NIST Standard Reference Database
- WebElements
- Sand Won't Save You This Time, blog post by Derek Lowe on the hazards of handling ClF3
Chlorine compounds Categories:- Fluorides
- Interhalogen compounds
- Inorganic chlorine compounds
- Incendiary weapons
- Rocket oxidizers
- Fluorinating agents
- Oxidizing agents
- F[Cl](F)F
Wikimedia Foundation. 2010.

