- PDE3 inhibitor
-
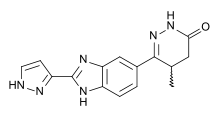
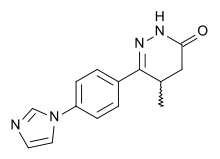
 Milrinone, one of the first PDE3 inhibitors used clinically
Milrinone, one of the first PDE3 inhibitors used clinically
A PDE3 inhibitor is a drug which inhibits the action of the phosphodiesterase enzyme PDE3. They are used for the therapy of acute heart failure and cardiogenic shock. Approved PDE3 inhibitors include amrinone, cilostazol, milrinone and enoximone. Pimobendane is approved for use in dogs.
Contents
Indications
PDE3 inhibitors are indicated as inotropics for the therapy of acute heart failure if catecholamines are ineffective.[1] Well controlled studies have shown that these drugs generally increase mortality,[2] so they have to be applied under close observation.[3]
PDE3 inhibitors are also used for the treatment of cardiogenic shock.[3]
Contraindications
Contraindications are severe obstructive cardiomyopathy, hypovolemia, tachycardia, and ventricular aneurysm. Breast feeding is prohibited during treatment.[3]
Adverse effects
The most important adverse effects are arrhythmia, thrombopenia and increased transaminase levels.[1][3]
Mechanism of action
PDE3 inhibitors are a type of phosphodiesterase inhibitors. Inhibition of the PDE isoenzyme 3 leads to an increase of intracellular concentrations of the second messenger cyclic adenosine monophosphate (cAMP). cAMP mediates the phosphorylation of protein kinases, which in turn activates cardiac calcium channels. An increased calcium influx from the sarcoplasmic reticulum (SR) during phase 2 (the plateau phase) of the cardiac action potential leads to a positive inotropic effect of PDE3 inhibitors: they increase the force of cardiac contraction. Inreased reflux of calcium into the SR following the plateau phase is responsible for their positive lusitropic effect: they increase relaxation speed. Additionally, PDE3 inhibitors act as vasodilators.[1][3]
Chemical properties
First generation PDE3 inhibitors
Recognition that the knowledge about PDE could be used to develop drugs that were PDE inhibitors led to extensive research. Most studies used analogues of the nucleotide substrates or derivatives of natural product inhibitors such as xanthine (e.g. theophylline) and papaverine.[4][5]
The active site of PDE3 can be considered as a summary of ideas about receptor topography resulting from the first generation inhibitors. The model of the Wells et al. version as cited in Erhardt and Chou (1991) includes the following:
- A phosphate binding area
- A lipophilic area that accommodates the non-polar side of the ribose moiety
- A pyrimidine binding site
- An imidazole binding site portion of the pyrimidine binding site
- A sterically hindered site
- An area with bulk tolerance[4]
Second generation PDE3 inhibitors
Since selective PDE3 inhibitors were recognised to be cardiotonic drugs there has been great interest in developing new drugs in this category. A large number of heterocyclic compounds have been synthesized during related research. These compounds constitute a second generation of PDE inhibitors. Although they have been directed mostly at PDE3, they present significant structure-activity relationship for the PDEs in general.[4]
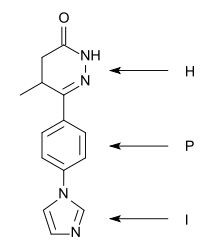 H (heterocycle) – P (phenyl) – I (imidazole) pattern of CI-930
H (heterocycle) – P (phenyl) – I (imidazole) pattern of CI-930
A “heterocycle-phenyl-imidazole” (H-P-I) pattern has been considered to be necessary for positive inotropic activity in cardiac muscle and many second generation inhibitors fit this pattern.[4]
The heterocycle region: Within each heterocycle there is the presence of a dipole and an adjacent acid proton (an amide function). These atoms are believed to mimic the electrophilic center in the phosphate group in cAMP and are confirmed as the primary site of binding. The heterocycle is a transition state analogue inhibitor of PDE. Alkyl groups, limited to either methyl or ethyl, on the heterocyclic ring usually enhance potency, with occasional exceptions.[4][5]
The phenyl region: It seems that an electron rich centre, such as phenyl, needs to be present. The beneficial effects of small alkyl groups on the heterocycle could be to twist the central ring away from exact coplanarity with the heterocyclic ring. There is a similar twist in cAMP and there is general agreement that high affinity PDE3 inhibitors should adopt an energetically favoured planar conformation that mimics the anti conformation of cAMP.[4][5]
The imidazole region: Various substituents have been placed at the para-position of the central phenyl ring. They are electron rich moieties and apparently a positively charged moiety cannot be tolerated in this region of the PDE receptor. There is general agreement about this inhibitor potency: lactam ≥ alkyl-CONH- ≥ imidazoyl = pyridine in place of the central phenyl with its nitrogen in the analogous 4 position ≥ alkyl-S- > simple ether > halide = amine > imidazolium (which is totally inactive).[4]
Identification of features common to the most selective inhibitors has led to a “five-point model” with:
- Presence of a strong dipole (carbonyl moiety) at one end of the molecule.
- An adjacent acid proton.
- A small sized alkyl substituent on the heterocyclic ring.
- A relatively flat overall topography.
- An electron rich centre and/or a hydrogen bond acceptor site opposite to the dipole.[5]
Examples of selective PDE3 inhibitors
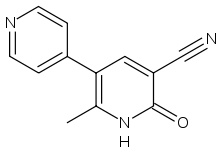
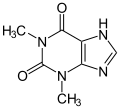 Theophylline, a non-selective inhibitor
Theophylline, a non-selective inhibitor
Theophylline is a non-selective agent. In contrast, meribendan is a highly selective inhibitor.[5]
Also, meribendan has a higher level of selectivity in comparison with the parent compound CI-930 because, beside the basic nitrogen adjacent to the lactam moiety it possesses another basic nitrogen (benzimidazole ring), opposite to the primary binding site.[5]
References
- ^ a b c Forth; Henschler; Rummel (2002) (in German). Allgemeine und spezielle Pharmakologie und Toxikologie. Munich. p. 457.
- ^ "Approval of Cilostazol". FDA. 25 September 2006. http://www.fda.gov/cder/news/cilostazol/approval.htm.[dead link]
- ^ a b c d e Mutschler, Ernst; Schäfer-Korting, Monika (1997) (in German). Arzneimittelwirkungen. Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 454–455, 496.
- ^ a b c d e f g Erhardt P.W., Chou Y. (1991). "A topographical model for the c-AMP phosphodiesterase III active site". Life Sciences, 49(8): 553-568.
- ^ a b c d e f Fossa P., Boggia R., Mosti L. (1998). "Toward the identification of the cardiac cGMP inhibited-phosphodiesterase catalytic site". Journal of Computer-Aided Molecular Design, 12(4): 361-372.
Phosphodiesterase inhibitors PDE1 MMPX • SCH-51866 • VinpocetinePDE2 BAY 60-7550 • EHNAPDE3 Amrinone • Anagrelide • Bucladesine • Cilostamide • Cilostazol • Enoximone • KMUP-1 • Milrinone • Quazinone • RPL-554 • Siguazodan • Trequinsin • Vesnarinone • ZardaverinePDE4 Arofylline • Cilomilast • CP-80633 • Denbutylline • Drotaverine • Etazolate • Filaminast • Glaucine • HT-0712 • Ibudilast • ICI-63197 • Irsogladine • Luteolin • Mesembrine • Roflumilast • Rolipram • Ro20-1724 • RPL-554 • YM-976PDE5 Acetildenafil • Aildenafil • Avanafil • Dipyridamole • Icariin • Lodenafil • Mirodenafil • MY-5445 • Sildenafil • Sulfoaildenafil • T-0156 • Tadalafil • Udenafil • VardenafilPDE6 ZaprinastPDE7 BRL-50481PDE9 BAY 73-6691 • SCH-51866PDE10 Nonselective Cardiac stimulants excluding cardiac glycosides (C01C) Adrenergic and
dopaminergic agentsαβmixedBothUnknown/ungroupedPhosphodiesterase inhibitors (PDE3I) Other cardiac stimulants #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Categories:- PDE3 inhibitors
Wikimedia Foundation. 2010.