[cite web | title = Entrez Gene: ALDOA aldolase A, fructose-bisphosphate| url = http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=226| accessdate = ] ] Complex Enzymatic Reaction
major_substrate_1=β-D-fructose 1,6-phosphate
major_substrate_1_stoichiometric_constant=
major_substrate_1_

major_substrate_2=
major_substrate_2_stoichiometric_constant=
major_substrate_2_
major_product_1=D-glyceraldehyde 3-phosphate
major_product_1_stoichiometric_constant=
major_product_1_
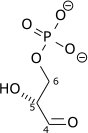
major_product_2=dihydroxyacetone phosphate
major_product_2_stoichiometric_constant=
major_product_2_

foward_enzyme=fructose bisphosphate aldolase
reverse_enzyme=fructose bisphosphate aldolase
reaction_direction_(foward/reversible/reverse)=reversible
minor_foward_substrate(s)=
minor_foward_product(s)=
minor_reverse_product(s)=
minor_reverse_substrate(s)=
"The numbering of the carbon atoms indicates the fate of the carbons according to their position in fructose 6-phosphate."
Mechanism
In mammalian aldolase the key catalytic amino acid residues involved in the reaction are lysine and tyrosine. The tyrosine acts as an efficient hydrogen acceptor while the lysine covalently binds and stabilizes the intermediates. Many bacteria use two magnesium ions in place of the lysine.
References
Further reading
PBB_Further_reading
citations =
*cite journal | author=Pfleiderer G, Thöner M, Wachsmuth ED |title=Histological examination of the aldolase monomer composition of cells from human kidney and hypernephroid carcinoma |journal=Beiträge zur Pathologie |volume=156 |issue= 3 |pages= 266–79 |year= 1976 |pmid= 766744 |doi=
*cite journal | author=Rehbein-Thöner M, Pfleiderer G |title=The changes in aldolase isoenzyme pattern during development of the human kidney and small intestine--demonstrated in organ extracts and tissue sections |journal=Hoppe-Seyler's Z. Physiol. Chem. |volume=358 |issue= 2 |pages= 169–80 |year= 1977 |pmid= 844801 |doi=
*cite journal | author=Wachsmuth ED |title=Differentiation of epithelial cells in human jejunum: localization and quantification of aminopeptidase, alkaline phosphatase and aldolase isozymes in tissue sections |journal=Histochemistry |volume=48 |issue= 2 |pages= 101–9 |year= 1976 |pmid= 955981 |doi=10.1007/BF00494548
*cite journal | author=Lee KN, Maxwell MD, Patterson MK, "et al." |title=Identification of transglutaminase substrates in HT29 colon cancer cells: use of 5-(biotinamido)pentylamine as a transglutaminase-specific probe |journal=Biochim. Biophys. Acta |volume=1136 |issue= 1 |pages= 12–6 |year= 1992 |pmid= 1353685 |doi=10.1016/0167-4889(92)90078-P
*cite journal | author=Dawson SJ, White LA |title=Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin |journal=J. Infect. |volume=24 |issue= 3 |pages= 317–20 |year= 1992 |pmid= 1602151 |doi=10.1016/S0163-4453(05)80037-4
*cite journal | author=Mukai T, Arai Y, Yatsuki H, "et al." |title=An additional promoter functions in the human aldolase A gene, but not in rat |journal=Eur. J. Biochem. |volume=195 |issue= 3 |pages= 781–7 |year= 1991 |pmid= 1999195 |doi=10.1111/j.1432-1033.1991.tb15766.x
*cite journal | author=Gamblin SJ, Davies GJ, Grimes JM, "et al." |title=Activity and specificity of human aldolases |journal=J. Mol. Biol. |volume=219 |issue= 4 |pages= 573–6 |year= 1991 |pmid= 2056525 |doi=10.1016/0022-2836(91)90650-U
*cite journal | author=Vértessy BG, Orosz F, Ovádi J |title=Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates |journal=Biochim. Biophys. Acta |volume=1078 |issue= 2 |pages= 236–42 |year= 1991 |pmid= 2065091 |doi=
*cite journal | author=Takasaki Y, Takahashi I, Mukai T, Hori K |title=Human aldolase A of a hemolytic anemia patient with Asp-128----Gly substitution: characteristics of an enzyme generated in E. coli transfected with the expression plasmid pHAAD128G |journal=J. Biochem. |volume=108 |issue= 2 |pages= 153–7 |year= 1990 |pmid= 2229018 |doi=
*cite journal | author=Gamblin SJ, Cooper B, Millar JR, "et al." |title=The crystal structure of human muscle aldolase at 3.0 A resolution |journal=FEBS Lett. |volume=262 |issue= 2 |pages= 282–6 |year= 1990 |pmid= 2335208 |doi=10.1016/0014-5793(90)80211-Z
*cite journal | author=Kishi H, Mukai T, Hirono A, "et al." |title=Human aldolase A deficiency associated with a hemolytic anemia: thermolabile aldolase due to a single base mutation |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=84 |issue= 23 |pages= 8623–7 |year= 1988 |pmid= 2825199 |doi=10.1073/pnas.84.23.8623
*cite journal | author=Izzo P, Costanzo P, Lupo A, "et al." |title=A new human species of aldolase A mRNA from fibroblasts |journal=Eur. J. Biochem. |volume=164 |issue= 1 |pages= 9–13 |year= 1987 |pmid= 3030757 |doi=10.1111/j.1432-1033.1987.tb10984.x
*cite journal | author=Inagaki H, Haimoto H, Hosoda S, Kato K |title=Aldolase C is localized in neuroendocrine cells |journal=Experientia |volume=44 |issue= 9 |pages= 749–51 |year= 1988 |pmid= 3046960 |doi=10.1007/BF01959149
*cite journal | author=Freemont PS, Dunbar B, Fothergill-Gilmore LA |title=The complete amino acid sequence of human skeletal-muscle fructose-bisphosphate aldolase |journal=Biochem. J. |volume=249 |issue= 3 |pages= 779–88 |year= 1988 |pmid= 3355497 |doi=
*cite journal | author=Izzo P, Costanzo P, Lupo A, "et al." |title=Human aldolase A gene. Structural organization and tissue-specific expression by multiple promoters and alternate mRNA processing |journal=Eur. J. Biochem. |volume=174 |issue= 4 |pages= 569–78 |year= 1988 |pmid= 3391172 |doi=10.1111/j.1432-1033.1988.tb14136.x
*cite journal | author=Maire P, Gautron S, Hakim V, "et al." |title=Characterization of three optional promoters in the 5' region of the human aldolase A gene |journal=J. Mol. Biol. |volume=197 |issue= 3 |pages= 425–38 |year= 1988 |pmid= 3441006 |doi=10.1016/0022-2836(87)90556-0
*cite journal | author=Kukita A, Yoshida MC, Fukushige S, "et al." |title=Molecular gene mapping of human aldolase A (ALDOA) gene to chromosome 16 |journal=Hum. Genet. |volume=76 |issue= 1 |pages= 20–6 |year= 1987 |pmid= 3570299 |doi=10.1007/BF00283044
*cite journal | author=Tolan DR, Niclas J, Bruce BD, Lebo RV |title=Evolutionary implications of the human aldolase-A, -B, -C, and -pseudogene chromosome locations |journal=Am. J. Hum. Genet. |volume=41 |issue= 5 |pages= 907–24 |year= 1987 |pmid= 3674018 |doi=
*cite journal | author=Sakakibara M, Mukai T, Hori K |title=Nucleotide sequence of a cDNA clone for human aldolase: a messenger RNA in the liver |journal=Biochem. Biophys. Res. Commun. |volume=131 |issue= 1 |pages= 413–20 |year= 1985 |pmid= 3840020 |doi=
*cite journal | author=Ovádi J, Mohamed Osman IR, Batke J |title=Interaction of the dissociable glycerol-3-phosphate dehydrogenase and fructose-1,6-bisphosphate aldolase. Quantitative analysis by an extrinsic fluorescence probe |journal=Eur. J. Biochem. |volume=133 |issue= 2 |pages= 433–7 |year= 1983 |pmid= 6406231 |doi=10.1111/j.1432-1033.1983.tb07482.x
External links
* http://pdbdev.sdsc.edu:48346/pdb/molecules/pdb50_5.html
*
*
*
PBB_Controls
update_page = yes
require_manual_inspection = no
update_protein_box = yes
update_summary = yes
update_citations = yes