- Chromium(III) acetylacetonate
-
Chromium(III) acetylacetonate 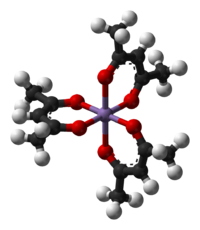 Chromium(III) acetylacetonateOther namestris(2,4-pentanediono)chromium(III)
Chromium(III) acetylacetonateOther namestris(2,4-pentanediono)chromium(III)Identifiers CAS number 21679-31-2 
ChemSpider 2006256 
ChEBI CHEBI:33035 
Jmol-3D images Image 1 - [Cr+3].O=C([CH-]C(=O)C)C.O=C([CH-]C(=O)C)C.O=C([CH-]C(=O)C)C
Properties Molecular formula C15H21CrO6 Molar mass 349.32 Appearance deep maroon Density 1.34 g/cm3 Melting point 216 °C
Boiling point 340 °C (sublimes near 100 °C)
Solubility in non-polar organic solvents soluble  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chromium(III) acetylacetonate is the coordination compound with the formula Cr(C5H7O2)3, sometimes designated as Cr(acac)3. This purplish coordination complex is used in NMR spectroscopy as a relaxation agent because of its solubility in nonpolar organic solvents and its paramagnetism.
Synthesis and structure
The compound is prepared by the reaction of chromium(III) oxide with acetylacetone (Hacac):[1]
- Cr2O3 + 6 Hacac → 2 Cr(acac)3 + 3 H2O
The complex has idealized D3 symmetry. Like other Cr(III) compounds, it has the d3 configuration, having a quartet ground state. Although it is relatively inert toward substitution, the complex undergoes bromination at the 3-positions of the chelate rings.
References
- ^ W. Conard Fernelius, Julian E. Blanch “Chromium(III) Acetylacetonate: [Tris(2,4-Pentanediono)Chromium(III)]” Inorganic Syntheses, 1957, Volume 5, 130-131.doi:10.1002/9780470132364.ch35
Categories:- Acetylacetonate complexes
- Organochromium compounds
Wikimedia Foundation. 2010.
