- Concanavalin A
-
Concanavalin A 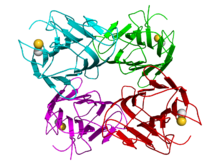
Crystallographic structure of a tetramer of jack bean concanavalin A (the monomers are colored cyan, green, red, and magenta respectively). Calcium (gold) and manganese cations (grey) are depicted as spheres.[1] Identifiers Organism Symbol ConA PDB 3CNA UniProt P81461 Other data Concanavalin A (ConA) is a lectin (carbohydrate-binding protein) originally extracted from the jack-bean, Canavalia ensiformis. It binds specifically to certain structures found in various sugars, glycoproteins, and glycolipids, mainly internal and nonreducing terminal α-D-mannosyl and α-D-glucosyl groups.[2][3] ConA is a plant mitogen, and is known for its ability to stimulate mouse T-cell subsets giving rise to four functionally distinct T cell populations, including precursors to suppressor T-cell;[4] one subset of human suppressor T-cells as well is sensitive to ConA.[4] ConA was the first lectin to be available on a commercial basis, and is widely used in biology and biochemistry to characterize glycoproteins and other sugar-containing entities on the surface of various cells.[5] It is also used to purify glycosylated macromolecules in lectin affinity chromatography,[6] as well as to study immune regulation by various immune cells.[4]
Contents
Structure and properties
As most lectins, the ConA is a homotetramer: each sub-unit (26.5KDa, 235 amino-acids, heavily glycated) binds a metallic atom (usually Mn2+ and a Ca2+). Its tertiary structure has been elucidated,[7] and molecular basis of its interactions with metals, its affinity for the mannose and glucose[8] are well known.
ConA binds specifically α-D-mannosyl and α-D-glucosyl residues (two hexoses differing only by the alcohol on carbon 2) in terminal position of ramified structures from B-Glycans (reach in α-mannose, or hybrid and bi-antennary glycanes complexes). It has 4 binding sites, corresponding to the 4 sub-units.[3] The molecular weight is 104-112KDa and the isoelectric point (pI) is in the range of 4.5-5.5.
Concanavalin A has a low-frequency wave number of 20 cm−1 in its Raman spectra.[9] This emission has been assigned to the breathing motion of the beta barrel consisting of 14 beta-strands in the concanavalin A molecule.[10]
ConA can also initiate cell division (mitogenesis) principally acting on T-lymphocytes, by stimulating the energy metabolism of thymocytes within seconds of exposure.[11]
For biotechnological uses, see Fluorescent glucose biosensors.Biological activity
Concanavalin A interacts with diverse receptors containing mannose carbohydrates, notably rhodopsin, blood group markers, insulin-receptor[12] the Immunoglobulins and the carcino-embryonary antigen (CEA). It also interacts with lipoproteins.[13]
ConA agglutinates strongly erythrocytes irrespective of blood-groups, and various cancerous cells.[14][15][16] It was demonstrated that transformed cells and trypsin-treated normal cells do not agglutinate at 4°C, thereby initiate suggesting that there is a temperature-sensitive step involved in ConA-mediated agglutination.[17][18]
ConA-mediated agglutination of other cell types has been reported, including muscle cells (myocytes),[19] B-lymphocytes (through surface Immunoglobulins),[20] fibroblasts,[21] rat thymocytes,[22] human fetal (but not adult) intestinal epithelial cells,[23] and adipocytes.[24] ConA is a also a lymphocyte mitogen.
ConA interacts with the surface mannose residues of many microbes, like the bacteria E. coli,[25] and Bacillus subtilis[26] and the protist Dictyostelium discoideum.[27]
It has also been shown as a stimulator of several matrix metalloproteinases (MMPs).[28]
ConA has proven useful in applications requiring solid-phase immobilization of glycoenzymes, especially those have proved difficult to immobilize by the traditional covalent coupling. Using ConA-couple matrices, such enzymes may be immobilized in high quantities without a concurrent loss of activity and/or stability. Such noncovalent ConA-glycoenzyme couplings may be relatively easily reversed by competition with sugars or at acidic pH. If necessary for certain applications, these couplings can be converted to covalent bindings by chemical manipulation.[29]
A recent (2009) report from Taiwan demonstrated potent therapeutic effect of ConA against experimental hepatoma (liver cancer); in the study by Lei and Chang,[30] ConA was found to be sequestered more by hepatic tumor cells, in preference to surrounding normal hepatocytes. Internalization of ConA occurs preferentially to the mitochondria after binding to cell membrane glycoproteins, which triggers an autophagic cell death. ConA was found to partially inhibit tumor nodule growth independent of its lymphocyte activation; the eradication of the tumor in the murine in situ hepatoma model in this study was additionally attributed to the mitogenic/lymphoproliferative action of ConA that may have activated a CD8+ T-cell-mediated, as well as NK- and NK-T cell-mediated, immune response in the liver.[30]
References
- ^ PDB 3CNA; Hardman KD, Ainsworth CF (December 1972). "Structure of concanavalin A at 2.4-A resolution". Biochemistry 11 (26): 4910–9. doi:10.1021/bi00776a006. PMID 4638345.
- ^ Goldstein IJ, Poretz RD (1986). "Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins". In Goldstein IJ, Liener IE, Sharon N. The Lectins Properties, Functions and Applications in Biology and Medicine. San Diego: Academic. pp. 233–247. ISBN 0-12-449945-7.
- ^ a b Sumner JB, Gralën N, Eriksson-Quensel IB (September 1938). "The molecular weights of canavalin, concanavalin A, and Concanavalin B". The Journal of Biological Chemistry 125 (1): 45–48. http://www.jbc.org/content/125/1/45.; Sumner, J. B.; Gralen, N.; Eriksson-Quensel, I.-B. (1938). "The Molecular Weights of Urease, Canavalin, Concanavalin a and Concanavalin B". Science 87 (2261): 395–396. doi:10.1126/science.87.2261.395. PMID 17746464.
- ^ a b c Dwyer JM, Johnson C (Nov 1981). "The use of concanavalin A to study the immunoregulation of human T cells". Clin Exp Immunol 46 (2): 237–49. PMC 1536405. PMID 6461456. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1536405.
- ^ Schiefer HG, Krauss H, Brunner H, Gerhardt U (Dec 1975). "Ultrastructural visualization of surface carbohydrate structures on mycoplasma membranes by concanavalin A". J Bacteriol 124 (3): 1598–600. PMC 236075. PMID 1104592. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=236075.
- ^ GE Healthcare Life Sciences, Immobilized lectin
- ^ Min W, Dunn AJ, Jones DH (April 1992). "Non-glycosylated recombinant pro-concanavalin A is active without polypeptide cleavage". EMBO J. 11 (4): 1303–7. PMC 556578. PMID 1563347. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=556578.
- ^ Loris R, Hamelryck T, Bouckaert J, Wyns L (March 1998). "Legume lectin structure". Biochim. Biophys. Acta 1383 (1): 9–36. doi:10.1016/S0167-4838(97)00182-9. PMID 9546043.
- ^ Painter PC, Mosher LE, Rhoads C (July 1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers 21 (7): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
- ^ Chou KC (August 1985). "Low-frequency motions in protein molecules. Beta-sheet and beta-barrel". Biophys. J. 48 (2): 289–97. Bibcode 1985BpJ....48..289C. doi:10.1016/S0006-3495(85)83782-6. PMC 1329320. PMID 4052563. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1329320.
- ^ Krauss S, Buttgereit F, Brand MD (June 1999). "Effects of the mitogen concanavalin A on pathways of thymocyte energy metabolism". Biochim Biophys Acta 1412 (2): 129–38. doi:10.1016/S0005-2728(99)00058-4. PMID 10393256.
- ^ http://www.pnas.org/content/70/2/485.short
- ^ J A Harmony and E H Cordes (1975); The Journal of Biological Chemistry, N.250. No.22, Issue 01, pp. 8614-8617. 1975; "Native human plasma low density lipoprotein (LDL) interacts with concanavalin A but not with ricin"
- ^ Betton GR (November 1976). "Agglutination reactions of spontaneous canine tumour cells, induced by concanavalin a, demonstrated by an isotopic assay". Int J Cancer 18 (5): 687–696. doi:10.1002/ijc.2910180518. PMID 992901.
- ^ Kakizoe T, Komatsu H, Niijima J, Kawachi T, Sugimura T (1980). "Increased agglutinability of bladder cells by concanavalin A after administration of carcinogens". Cancer Res 40 (6): 2006–2009. PMID 7371036.
- ^ Becker FF, Shurgin A (October 1975). "Concanavalin A Agglutination of Cells from Primary Hepatocellular Carcinomas and Hepatic Nodules Induced by N-2-Fluorenylacetamide" (PDF). Cancer Res 35 (10): 2879. PMID 168971. http://cancerres.aacrjournals.org/content/35/10/2879.full.pdf.
- ^ INBAR M, BEN-BASSAT H, SACHS K (1971). "A specific membrane activity on the surface membrane in malignant cell transformation". Proc Natl Acad Sci, USA 68 (11): 2748. doi:10.1073/pnas.68.11.2748. PMC 389516. PMID 4330939. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=389516.
- ^ SELA B, Lis H, SHARON N, SACHS L (1971). "Quantitation of N-acetyl-D-galactosamine sites on the surface membrane of normal and transformed cells". Biochim . Biophys. Acta 249: 564.
- ^ Gartner TK, Podleski TR (Dec 1975). "Evidence that a membrane bound lectin mediates fusion of L6 myoblasts". Biochem Biophys Res Commun 67 (3): 972–8. doi:10.1016/0006-291X(75)90770-6. PMID 1201086.
- ^ de Petris S (April 1975). "Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures". J Cell Biol 65 (1): 123–146. doi:10.1083/jcb.65.1.123. PMC 2111157. PMID 1092699. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2111157.
- ^ Noonan KD, Burger MM (october 1973). "THE RELATIONSHIP OF CONCANAVALIN A BINDING TO LECTIN-INITIATED CELL AGGLUTINATION". J Cell Biol 59 (1): 134–142. doi:10.1083/jcb.59.1.134. PMC 2110924. PMID 4201706. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2110924.
- ^ Capo C, Garrouste F, Benoliel AM, Bongrand P, Ryter A, Bell GI (Aug 1982). "Concanavalin-A-mediated thymocyte agglutination: a model for a quantitative study of cell adhesion". J Cell Sci 56: 21–48. PMID 7166565.
- ^ Weiser MM (Aug 1972). "Concanavalin A agglutination of intestinal cells from the human fetus". Science 177 (4048): 525–6. doi:10.1126/science.177.4048.525. PMID 5050484.
- ^ Cuatrecasas P (March 1973). "Interaction of wheat germ agglutinin and concanavalin A with isolated fat cells". Biochemistry 12 (7): 1312–1323. doi:10.1021/bi00731a011. PMID 4696755.
- ^ OFEK I, MIRELMAN D, SHARON N (February 1977). "Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors". Nature 265 (5595): 623–625. doi:10.1038/265623a0. PMID 323718.
- ^ Doyle RJ, Birdsell DC (Feb 1972). "Interaction of concanavalin A with the cell wall of Bacillus subtilis". J Bacteriol 109 (2): 652–8. PMC 285189. PMID 4621684. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=285189.
- ^ West CM, McMahon D (July 1977). "Identification of concanavalin A receptors and galactose-binding proteins in purified plasma membranes of Dictyostelium discoideum". J Cell Biol 74 (1): 264–273. doi:10.1083/jcb.74.1.264. PMC 2109878. PMID 559679. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2109878.
- ^ Yu M, Sato H, Seiki M, Thompson EW (August 1995). "Complex regulation of membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation by concanavalin A in MDA-MB-231 human breast cancer cells". Cancer Res. 55 (15): 3272–7. PMID 7614461. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=7614461.
- ^ Saleemuddin M, Husain Q (April 1991). "Concanavalin A: A useful ligand for glycoenzyme immobilization--A review". Enzyme and Microbial Technology 13 (4): 290–295. doi:10.1016/0141-0229(91)90146-2. PMID 1367163. http://www.sciencedirect.com/science/article/B6TG1-47DM5XT-12T/2/405a7087bf0f3daef3026681bf7c5658.
- ^ a b Lei HY, Chang CP (Jan 2009). "Lectin of Concanavalin A as an anti-hepatoma therapeutic agent". J Biomed Sci 16 (1): 10. doi:10.1186/1423-0127-16-10. PMC 2644972. PMID 19272170. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2644972.
External links
- MeSH Concanavalin+A
- MeSH Concanavalin+A+Receptors
- Concanavalin A structure
- World of Lectin, Gateway to lectins
- Proteopedia 1bxh con A in complex with methyl alpha1-2 mannobioside
Animal C-type lectinsAsialoglycoprotein receptor · KLRD1 · Collectin (Mannan-binding lectin) · Mannose receptor · proteochondroitin sulfate (Aggrecan, Versican, Brevican, Neurocan)OtherPlant Abrin · Ricin · Mitogens (Concanavalin A, Phytohaemagglutinin, Pokeweed mitogen) · Legume lectin · BanLecCategories:- Proteins
- Lectins
- Legume Lectins
Wikimedia Foundation. 2010.
