- Trimethylamine
-
Trimethylamine[1] 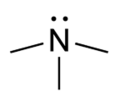
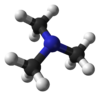
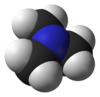 TrimethylamineOther namesN,N-Dimethylmethanamine
TrimethylamineOther namesN,N-DimethylmethanamineIdentifiers Abbreviations TMA, NMe3 CAS number 75-50-3 
PubChem 1146 ChemSpider 1114 
UNII LHH7G8O305 
EC number 200-875-0 UN number 1083 (anhydrous)
1297 (solution)ChEBI CHEBI:18139 
ChEMBL CHEMBL439723 
RTECS number 1.1.78 – 31.1.87 Jmol-3D images Image 1 - N(C)(C)C
Properties Molecular formula C3H9N Molar mass 59.11 g/mol Appearance Colorless gas Density 670 g/L (0 °C) Melting point -117.08 °C, 156 K, -179 °F
Boiling point 2.87 °C, 276 K, 37 °F
Solubility in water Miscible Acidity (pKa) 9.76[2] Hazards R-phrases R12 R20/22 R34 S-phrases (S1/2) S3 S16 S26 S29 S36/37/39 S45 NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trimethylamine is an organic compound with the formula N(CH3)3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations. It is a gas at room temperature but is usually sold in pressurized gas cylinders or as a 40% solution in water.
Trimethylamine is a product of decomposition of plants and animals. It is the substance mainly responsible for the odor often associated with fouling fish, some infections, and bad breath. It is also associated with taking large doses of choline and carnitine.
Trimethylamine is a nitrogenous base and can be readily protonated to give trimethylammonium cation. Trimethylammonium chloride is a hygroscopic colorless solid prepared from hydrochloric acid. Trimethylamine is a good nucleophile, and this reaction is the basis of most of its applications.
Contents
Production
Trimethylamine is prepared by the reaction of ammonia and methanol employing a catalyst:[3]
- 3 CH3OH + NH3 → (CH3)3N + 3 H2O
This reaction coproduces the other methylamines, dimethylamine (CH3)2NH and methylamine CH3NH2.
Trimethylamine has also been prepared via a reaction of ammonium chloride and paraformaldehyde,[4] according to the following equation:
- 9 (CH2=O)n + 2n NH4Cl → 2n (CH3)3N•HCl + 3n H2O + 3n CO2↑
Applications
Trimethylamine is used in the synthesis of choline, tetramethylammonium hydroxide, plant growth regulators, strongly basic anion exchange resins, dye leveling agents and a number of basic dyes.[3][5] Gas sensors to test for fish freshness detect trimethylamine.
Trimethylaminuria
Main article: TrimethylaminuriaTrimethylaminuria is a genetic disorder in which the body is unable to metabolize trimethylamine from food sources. Patients develop a characteristic fish odour of their sweat, urine, and breath after the consumption of choline-rich foods. Trimethylaminuria is an autosomal recessive disorder involving a trimethylamine oxidase deficiency. A condition similar to trimethylaminuria has also been observed in a certain breed of Rhode Island Red chicken that produces eggs with a fishy smell, especially after eating food containing a high proportion of rapeseed.[citation needed]
See also
- Ammonia, NH3
- Ammonium, NH4+
- Methylamine (CH3)NH2
- Dimethylamine (CH3)2NH
- Triethylamine (TEA)
References
- ^ Merck Index, 11th Edition, 9625.
- ^ Hall, H.K. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79: 5441. doi:10.1021/ja01577a030.
- ^ a b A. B. van Gysel, W. Musin "Methylamines" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. doi:10.1002/14356007.a16 535
- ^ Roger Adams, B. K. Brown, "Trimethylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0531; Coll. Vol. 1: 75
- ^ Ashford's Dictionary of Industrial Chemicals (3rd ed.). 2011. p. 9362. ISBN 978-0-9522674-3-0.
External links
Categories:- Amines
- Foul-smelling chemicals
Wikimedia Foundation. 2010.

