- Dimethylheptylpyran
-
Dimethylheptylpyran 
Systematic (IUPAC) name 6,6,9-trimethyl-3-(3-methyloctan-2-yl)- Clinical data Pregnancy cat. ? Legal status Schedule I / Class B Pharmacokinetic data Half-life 20-39 hours Identifiers CAS number 32904-22-6 ATC code None PubChem CID 36276 ChemSpider 19960038 
Chemical data Formula C25H38O2 Mol. mass 370.57 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dimethylheptylpyran (1,2-dimethylheptyl-Δ3THC, DMHP, A-40824, EA-1476) is a synthetic analogue of THC, which was invented in 1949 during attempts to elucidate the structure of Δ9-THC, the active component of cannabis.[1] DMHP is a pale yellow, viscous oil which is insoluble in water, but dissolves in alcohol or non-polar solvents.
Effects
DMHP is similar in structure to THC, differing only in the position of one double bond, and the replacement of the 3-pentyl chain with a 3-(1,2-dimethylheptyl) chain.[2] It produces similar activity to THC, such as sedative effects, but is considerably more potent,[3] especially having much stronger analgesic and anticonvulsant effects than THC, although comparatively weaker psychological effects. It is thought to act as a CB1 agonist, in a similar manner to other similar cannabinoid derivatives.[4]
Investigation as non-lethal incapacitating agent
DMHP and its O-acetate ester were extensively investigated by the US military chemical weapons program in the Edgewood Arsenal experiments, as possible non-lethal incapacitating agents.[5]
DMHP has three stereocenters and consequently has eight possible stereoisomers, which differ considerably in potency. The racemic mix of all eight isomers of the O-acetyl ester was given the code number EA-2233, with the eight individual isomers numbered EA-2233-1 through EA-2233-8. The most potent isomer was EA-2233-2, with an active dose range in humans of 0.5-2.8 μg/kg (i.e. ~35-200 μg for a 70 kg adult). Active doses varied markedly between individuals, but when the dose of EA-2233 was taken up to 1–2 mg, all volunteers were considered to be incapable of performing military duties, with the effects lasting as long as 2–3 days.
DMHP is metabolised in a similar manner to THC, producing the active metabolite 11-hydroxy-DMHP, but the lipophilicity of DMHP is even higher than that of THC itself, giving it a long duration of action and an extended half-life in the body of between 20–39 hours, with the half-life of the 11-hydroxy-DMHP metabolite being longer than 48 hours.
Cannabinoids as a class are generally safe compounds with a large safety margin, making potent cannabinoid drugs ideal as potential non-lethal incapacitating agents. DMHP and its esters produce sedation and mild hallucinogenic effects similar to large doses of THC, but in addition to this they also cause pronounced hypotension (low blood pressure) which occurs at doses well below the hallucinogenic dose, and can lead to severe dizziness, fainting, ataxia and muscle weakness, sufficient to make it difficult to stand upright or carry out any kind of vigorous physical activity. The acute toxicity of DMHP was found to be low in both human and animal studies, with the ratio of ED50 to LD50 in animals being around 2000x, with death ultimately resulting from a combination of hypotension and hypothermia and preventable with supportive treatment.
The combination of strong incapacitating effects and a favourable safety margin led the Edgewood Arsenal team to conclude that DMHP and its derivatives, especially the O-acetyl ester of the most active isomer, EA-2233-2, were among the more promising non-lethal incapacitating agents to come out of their research program. However they were disadvantaged by producing severe hypotension at incapacitating doses, and were not as effective as the more widely publicised anticholinergic agents such as 3-Quinuclidinyl benzilate which had also already been weaponised.[6] Funding for continued development was ultimately not approved, and the cannabinoid research program was indefinitely suspended along with the rest of the Edgewood Arsenal experiments in the late 1970s, in accordance with the US commitment to cease research into chemical weapons under disarmament treaties.
References
- ^ Adams R, Harfenist M, Loewe S. New Analogs of Tetrahydrocannabinol. XIX. Journal of the American Chemical Society. 1949; 71(5):1624-1628.
- ^ Razdan RK. The Total Synthesis of Cannabinoids. Wiley-Interscience 1980.
- ^ Wilkison, DM; Pontzer, N; Hosko, MJ (1982). "Slowing of cortical somatosensory evoked activity by delta 9-tetrahydrocannabinol and dimethylheptylpyran in alpha-chloralose-anesthetized cats". Neuropharmacology 21 (7): 705–9. doi:10.1016/0028-3908(82)90014-4. PMID 6289158.
- ^ Parker, LA; Mechoulam, R (2003). "Cannabinoid agonists and antagonists modulate lithium-induced conditioned gaping in rats". Integrative physiological and behavioral science : the official journal of the Pavlovian Society 38 (2): 133–45. PMID 14527182.
- ^ Possible Long-Term Health Effects of Short-Term Exposure To Chemical Agents, Volume 2: Cholinesterase Reactivators, Psychochemicals and Irritants and Vesicants (1984) Commission on Life Sciences. The National Academies Press. pp79-99.
- ^ James S. Ketchum. Chemical Warfare Secrets Almost Forgotten. ChemBooks Inc. 2006. ISBN 978-1-4243-0080-8
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesNaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
Others(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone · N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide · A-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · N-arachidonoyl-serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control 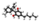
This cannabinoid related article is a stub. You can help Wikipedia by expanding it.
