- Chromium trioxide
-
Chromium trioxide 
 Chromium trioxide
Chromium trioxide
Chromium(VI) oxideOther namesChromic anhydride, chromium(VI) oxide, chromic acid, anhydride, chromic acid (misnomer)Identifiers CAS number 1333-82-0 
PubChem 14915 ChemSpider 14212 
UNII 8LV49809UC 
UN number 1463 ChEBI CHEBI:48240 
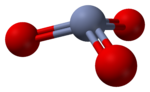
RTECS number GB6650000 Jmol-3D images Image 1 - O=[Cr](=O)=O
Properties Molecular formula CrO3 Molar mass 99.99 g mol−1 Appearance dark red granular solid Odor odorless Density 2.70 g/cm3 (20 °C) Melting point 197 °C, 470 K, 387 °F
Boiling point 251 °C, 524 K, 484 °F (decomposes)
Solubility in water 61.7 g/100 mL (0 °C)
63 g/100 mL (25 °C)
67 g/100 mL (100 °C)Solubility soluble in sulfuric acid, nitric acid Hazards MSDS ICSC 1194 EU Index 024-001-00-0 EU classification Oxidizer (O)
Carc. Cat. 1
Muta. Cat. 2
Repr. Cat. 3
Very toxic (T+)
Dangerous for the environment (N)R-phrases R45, R46, R9, R24/25, R26, R35, R42/43, R48/23, R62, R50/53 S-phrases S53, S45, S60, S61 NFPA 704 LD50 80 mg/kg  trioxide (verify) (what is:
trioxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.[1] This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating.[2]
Contents
Production, structure, and basic reactions
Chromium trioxide is generated by treating sodium chromate or the corresponding sodium dichromate with sulfuric acid:[1]
- H2SO4 + Na2CrO4 → CrO3 + Na2SO4 + H2O
Approximately 100M kg are produced annually by this or similar routes.[2]
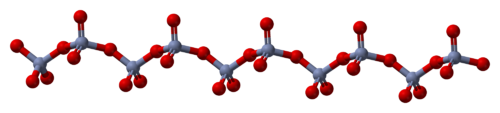
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center, therefore, shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.[3][4]
The structure of monomeric CrO3 has been calculated using density functional theory, and is predicted to be pyramidal (point group C3v) rather than planar (point group D3h).[5]
Chromium trioxide decomposes above 197°C liberating oxygen eventually giving Cr2O3:
- 4 CrO3 → 2 Cr2O3 + 3 O2
It is used in organic synthesis as an oxidant, often as a solution in acetic acid,[3] or acetone in the case of the Jones oxidation. In these oxidations, the Cr(VI) converts 1.5 equivalents of alcohols to the corresponding ketones or aldehydes:
- 2 CrO3 + 3 RCH2OH → Cr2O3 + 3 RCHO + 3 H2O
Applications
Chromium trioxide is mainly used in chrome-plating. It is typically employed with additives that affect the plating process but do not react with the trioxide. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. It is also used in the production of synthetic rubies.
Safety
Chromium trioxide is highly toxic, corrosive, and carcinogenic.[6] It is the main example of hexavalent chromium, an environmental hazard. The related chromium(III) derivatives are not particularly dangerous; thus, reductants are used to destroy chromium(VI) samples.
Chromium trioxide, being a powerful oxidizer, will ignite some organic materials (such as ethanol) on contact.
References
- ^ a b "Chromium Trioxide". Chemicalland21. http://www.chemicalland21.com/industrialchem/inorganic/CHROMIUM%20TRIOXIDE.htm.
- ^ a b Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinge. Chromium Compounds. in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, 2002. doi:10.1002/14356007.a07_067
- ^ a b Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ Stephens, J. S.; Cruickshank, D. W. J. (1970). "The crystal structure of (CrO3)∞". Acta Cryst. B 26: 222–226. doi:10.1107/S0567740870002182.
- ^ Zhai, Hua-Jin; Li, Shenggang; Dixon, David A.; Wang, Lai-Sheng (2008). "Probing the Electronic and Structural Properties of Chromium Oxide Clusters (CrO3)n− and (CrO3)n (n = 1–5): Photoelectron Spectroscopy and Density Functional Calculations". J. Am. Chem. Soc. 130 (15): 5167–5177. doi:10.1021/ja077984d.
- ^ "Chromium Trioxide (MSDS)". J. T. Baker. http://hazard.com/msds/mf/baker/baker/files/c4400.htm. Retrieved 2007-09-13.
External links
- ATSDR Case Studies in Environmental Medicine: Chromium Toxicity U.S. Department of Health and Human Services
- http://www.gemstonebuzz.com/flame-fusion
Chromium compounds Categories:- Oxides
- Chromium compounds
Wikimedia Foundation. 2010.