- Neural crest
-
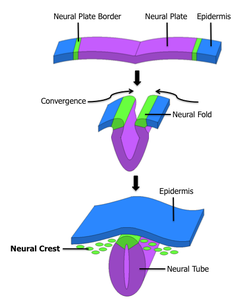
Neural crest The formation of neural crest during the process of neurulation. Neural crest is first induced in the region of the neural plate border. After neural tube closure, neural crest delaminates from the region between the dorsal neural tube and overlying ectoderm and migrates out towards the periphery. Latin crista neuralis Code TE E5.0.2.1.0.0.2 Neural crest cells are a transient, multipotent, migratory cell population unique to vertebrates that gives rise to a diverse cell lineage including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia.[1]
After gastrulation, neural crest cells are specified at the border of the neural plate and the non-neural ectoderm. During neurulation, the borders of the neural plate, also known as the neural folds, converge at the dorsal midline to form the neural tube. Subsequently, neural crest cells from the roof plate of the neural tube undergo an epithelial to mesenchymal transition, delaminating from the neuroepithelium and migrating through the periphery where they differentiate into varied cell types.[1] The emergence of neural crest was important in vertebrate evolution because many of its structural derivatives are defining features of the vertebrate clade.[2]
Underlying the development of neural crest is a gene regulatory network, described as a set of interacting signals, transcription factors, and downstream effector genes that confer cell characteristics such as multipotency and migratory capabilities.[3] Understanding the molecular mechanisms of neural crest formation is important for our knowledge of human disease because of its contributions to multiple cell lineages. Abnormalities in neural crest development cause neurocristopathies, which include conditions such as frontonasal dysplasia, Waardenburg-Shah syndrome, and DiGeorge syndrome.[1]
Therefore, defining the mechanisms of neural crest development may reveal key insights into vertebrate evolution and neurocristopathies.
Contents
History
Neural crest was first described in the chick embryo by Wilhelm His in 1868 as "the cord in between" (Zwischenstrang) because of its origin between the neural plate and non-neural ectoderm.[1] He named the tissue ganglionic crest since its final destination was each lateral side of the neural tube where it differentiated into spinal ganglia.[4] During the first half of the 20th century the majority of research on neural crest was done using amphibian embryos which was reviewed by Hörstadius (1950) in a well known monograph.[5]
Cell labeling techniques advanced the field of neural crest because they allowed researchers to visualize the migration of the tissue throughout the developing embryos. In the 1960s Weston and Chibon utilized radioisotopic labeling of the nucleus with tritiated thymidine in chick and amphibian embryo respectively. However, this method suffers from drawbacks of stability, since every time the labeled cell divides the signal is diluted. Modern cell labeling techniques such as rhodamine-lysinated dextran and the vital dye diI have also been developed to transiently mark neural crest lineages.[4]
The quail-chick marking system, devised by Nicole Le Douarin in 1969, was another instrumental technique used to track neural crest cells.[6] [7] Chimeras, generated through transplantation, enabled researchers to distinguish neural crest cells of one species from the surrounding tissue of another species. With this technique, generations of scientists were able to reliably mark and study the ontogeny of neural crest cells.
 Putative neural crest gene-regulatory network functioning at the neural plate border in vertebrates. Red arrows represent proven direct regulatory interactions. Black arrows show genetic interactions based on loss-of-function and gain-of-functions studies. Gray lines denote repression. Adapted from Bronner-Fraser 2004.
Putative neural crest gene-regulatory network functioning at the neural plate border in vertebrates. Red arrows represent proven direct regulatory interactions. Black arrows show genetic interactions based on loss-of-function and gain-of-functions studies. Gray lines denote repression. Adapted from Bronner-Fraser 2004.
Induction
A molecular cascade of events is involved in establishing the migratory and multipotent characteristics of neural crest cells. This gene-regulatory network can be subdivided into the following four sub-networks described below.
Inductive signals
First, extracellular signaling molecules, secreted from the adjacent epidermis and underlying mesoderm such Wnts, BMPs and Fgfs separate the non-neural ectoderm (epidermis) from the neural plate during neural induction.[1][2]
Wnt signaling has been demonstrated in neural crest induction in several species through gain-of-function and loss-of-function experiments. In coherence with this observation, the promoter region of slug (a neural crest specific gene) contains a binding site for transcription factors involved in the activation of Wnt-dependent target genes, suggestive of a direct role of Wnt signaling in neural crest specification.[8]
The current role of BMP in neural crest formation is associated with the induction of the neural plate. BMP antagonists diffusing from the ectoderm generates a gradient of BMP activity. In this manner, the neural crest lineage forms from intermediate levels of BMP signaling required for the development of the neural plate (low BMP) and epidermis (high BMP).[1]
Fgf from the paraxial mesoderm has been suggested as a source of neural crest inductive signal. Researchers have demonstrated that the expression of dominate-negative Fgf receptor in ectoderm explants blocks neural crest induction when recombined with paraxial mesoderm.[9] Our current understanding of the role of BMP, Wnt, and Fgf pathways on neural crest specifier expression remains incomplete.
Neural plate border specifiers
Signaling events that establish the neural plate border lead to the expression of a set of transcription factors delineated here as neural plate border specifiers. These molecules include Zic factors, Pax3/7, Dlx5, Msx1/2 which may mediate the influence of Wnts, BMPs, and Fgfs. These genes are expressed broadly at the neural plate border region and precede the expression of bona fide neural crest markers.[2]
Experimental evidence places these transcription factors upstream of neural crest specifiers. For example, in Xenopus Msx1 is necessary and sufficient for the expression of Slug, Snail, and FoxD3.[10] Furthermore, Pax3 is essential for FoxD3 expression in mouse embryos.[11]
Neural crest specifiers
Following the expression of neural plate border specifiers is a collection of genes including Slug/Snail, FoxD3, Sox10, Sox9, AP-2 and c-Myc. This suite of genes, designated here as neural crest specifiers, are activated in emergent neural crest cells. At least in Xenopus, every neural crest specifier is necessary and/or sufficient for the expression of all other specifiers, demonstrating the existence of extensive cross-regulation.[2]
Outside of the tightly regulated network of neural crest specifiers are two other transcription factors Twist and Id. Twist, a bHLH transcription factor, is required for mesenchyme differentiation of the pharyngeal arch structures.[12] Id is a direct target of c-Myc and is known to be important for the maintenance of neural crest stem cells.[13]
Neural crest effector genes
Finally, neural crest specifiers turn on the expression of effector genes, which confer certain properties such as migration and multipotency. Two neural crest effectors, Rho GTPases and cadherins, function in delamination by regulating cell morphology and adhesive properties. Sox9 and Sox10 regulate neural crest differentiation by activating many cell-type-specific effectors including Mitf, P0, Cx32, Trp and cKit.[2]
Cell lineages
Neural crest cells originating from different positions along the anterior-posterior axis develop into various tissues. These regions of neural crest can be divided into four main functional domains, which include the cranial neural crest, trunk neural crest, vagal and sacral neural crest, and cardiac neural crest.
Cranial neural crest
Main article: cranial neural crestCranial neural crest migrates dorsolaterally to form the craniofacial mesenchyme that differentiates into various cranial ganglia and craniofacial cartilages and bones.[14] These cells enter the pharyngeal pouches and arches where they contribute to the thymus, bones of the middle ear and jaw and the odontoblasts of the tooth primordia.[15]
Trunk neural crest
Main article: trunk neural crestTrunk neural crest gives rise to two populations of cells. One group of cells fated to become melanocytes migrates dorsolaterally into the ectoderm towards the ventral midline. A second group of cells migrates ventrolaterally through the anterior portion of each sclerotome. The cells that stay in the sclerotome form the dorsal root ganglia, whereas those that continue more ventrally form the sympathetic ganglia, adrenal medulla, and the nerves surrounding the aorta.[15]
Vagal and sacral neural crest
The vagal and sacral neural crest cells develop into the ganglia of the enteric nervous system, also known as the parasympathetic ganglia.[15]
Cardiac neural crest
Main article: cardiac neural crestCardiac neural crest develops into melanocytes, cartilage, connective tissue and neurons of some pharyngeal arches. Also, this domain gives rise to regions of the heart such as the musculo-connective tissue of the large arteries, and part of the septum, which divides the pulmonary circulation from the aorta.[15] The semilunar valves of the heart are associated with neural crest cells according to new research.[16]
Evolution
Several structures that distinguish the vertebrates from other chordates are formed from the derivatives of neural crest cells. In Gans and Northcut's "New head" theory they argued that the presence of neural crest was the basis for vertebrate specific features, such as sensory ganglia and cranial skeleton. Furthermore, the appearance of these features was pivotal in vertebrate evolution because it enabled a predatory lifestyle.[17]
However, considering the neural crest a vertebrate innovation does not mean that it was created de novo. Instead, new structures often arise through modification of existing developmental regulatory programs. For example, regulatory programs may be changed by the co-option of new upstream regulators or by the employment of new downstream gene targets, thus placing existing networks in a novel context.[18][19] This idea is supported by in situ hybridization data that shows the conservation of the neural plate border specifiers in protochordates, which suggest that part of the neural crest precursor network was present in a common ancestor to the chordates.[3]
Neural Crest derivatives
Mesectoderm[20] : odontoblasts, dental papillae, the chondrocranium (nasal capsule, Meckel's cartilage, scleral ossicles, quadrate, articular, hyoid and columella), tracheal and laryngeal cartilage, the dermatocranium (membranous bones), dorsal fins and the turtle plastron (lower vertebrates), pericytes and smooth muscle of branchial arteries and veins, tendons of ocular and masticatory muscles, connective tissue of head and neck glands (pituitary, salivary, lachrymal, thymus, thyroid) dermis and adipose tissue of calvaria, ventral neck and face
Endocrine Cells: enterochromaffin, parafollicular cells of the thyroid, carotid body type I/II, adrenal medulla
Peripheral nervous system: Sensory neurons and glia of the dorsal root ganglia, cephalic ganglia (VII and in part, V, IX, and X), Rohon-Beard cells, Merkel cells [21], Satellite glial cells of all autonomic and sensory ganglia, Schwann cells of all peripheral nerves
Melanocytes and iris pigment cells
See also
- Neural plate
- DGCR2 - may control neural crest cell migration
References
- ^ a b c d e f Huang, X., and Saint-Jeannet, J.P. (2004). "Induction of the neural crest and the opportunities of life on the edge". Dev. Biol. 275, 1-11.doi:10.1016/j.ydbio.2004.07.033
- ^ a b c d e Meulemans, D., and Bronner-Fraser, M. (2004). "Gene-regulatory interactions in neural crest evolution and development". Dev Cell. 7, 291-9.doi:10.1016/j.devcel.2004.08.007
- ^ a b Sauka-Spengler, T., Meulemans, D., Jones, M., and Bronner-Fraser, M. (2007). "Ancient evolutionary origin of the neural crest gene regulatory network". Dev Cell. 13, 405-20. doi:10.1016/j.devcel.2007.08.005 PMID 17765683
- ^ a b Le Douarin, N.M. (2004). "The avian embryo as a model to study the development of the neural crest: a long and still ongoing story". Mech Dev. 121, 1089-102. doi:10.1016/j.mod.2004.06.003
- ^ Hörstadius, S. (1950). The Neural Crest: Its Properties and Derivatives in the Light of Experimental Research. Oxford University Press, London, 111 p.
- ^ Le Douarin, N.M. (1969). "Particularités du noyau interphasique chez la Caille japonaise (Coturnix coturnix japonica). Utilisation de ces particularités comme «marquage biologique» dans les recherches sur les interactions tissulaires et les migrations cellulaires au cours de l'ontogenèse". Bull biol Fr Belg 103 : 435-52.
- ^ Le Douarin, N.M. (1973). "A biological cell labeling technique and its use in experimental embryology". Dev Biol. 30 217-22. doi:10.1016/0012-1606(73)90061-4
- ^ Vallin, J. et al. (2001). "Cloning and characterization of the three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling". J Biol Chem. 276, 30350-8. doi:10.1074/jbc.M103167200
- ^ Mayor, R., Guerrero, N., Martinez, C. (1997). "Role of FGF and noggin in neural crest induction". Dev Biol. 189 1-12. doi:10.1006/dbio.1997.8634
- ^ Tribulo, C. et al. (2003). "Regulation of Msx genes by Bmp gradient is essential for neural crest specification". Development. 130, 6441-52.doi:10.1242/dev.00878
- ^ Dottori, M., Gross, M.K., Labosky, P., and Goulding, M. (2001). "The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate". Development 128, 4127–4138.
- ^ Vincentz, J.W. et al. (2008). "An absence of Twist1 results in aberrant cardiac neural crest morphogenesis". Dev Biol. 320, 131-9. doi:10.1016/j.ydbio.2008.04.037
- ^ Light, W. et al. (2005). "Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells". Development. 132, 1831-41. doi: 10.1242/dev.01734
- ^ Taneyhill, L.A. (2008). "To adhere or not to adhere: the role of Cadherins in neural crest development". Cell Adh Migr. 2, 223-30.
- ^ a b c d http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=dbio&part=A3109#A3133
- ^ http://www.springerlink.com/content/h47w315112064434/
- ^ Gans, C. and Northcutt, R. G. (1983). "Neural crest and the origin of vertebrates: A new head". Science 220, 268–274. doi:10.1126/science.220.4594.268
- ^ Sauka-Spengler, T. and Bronner-Fraser, M. (2006). "Development and evolution of the migratory neural crest: a gene regulatory perspective". Curr Opin Genet Dev. 13, 360-6. doi:10.1016/j.gde.2006.06.006
- ^ Donoghue, P.C., Graham, A., Kelsh, R.N. (2008). "The origin and evolution of the neural crest". Bioessays. 30, 530-41. doi:10.1002/bies.20767
- ^ Kalcheim, C. and Le Douarin, N. M. (1998). The Neural Crest (2nd ed.). Cambridge, U. K.: Cambridge University Press.
- ^ Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev Biol. 2003 Jan 15;253(2):258-63. PubMed PMID: 12645929.
External links
- Embryology at UNSW Notes/ncrest
- NeuroNames ancil-445
- Diagram at University of Michigan
- Hox domains in chicks
Developmental biology > Human embryogenesis (development of embryo) and development of fetus (TE E2.0) First three
weeksWeek 1Fertilization · Oocyte activation · Zygote · Cleavage · Morula · Blastula (Blastomere) · Blastocyst · Inner cell massWeek 2
(Bilaminar)Week 3
(Trilaminar)Archenteron/Primitive streak (Primitive pit, Primitive knot/Blastopore, Primitive groove) · Gastrula/Gastrulation · Regional specification · Embryonic discSplanchnopleuric mesenchymeChorda- · Paraxial (Somite/Somitomere) · Intermediate · Lateral plate (Intraembryonic coelom, Splanchnopleuric mesenchyme/Somatopleuric mesenchyme)Prenatal development/Mammalian development of nervous system (GA 9.733 and GA 10.1002, TE E5.13-16) Neurogenesis Neural crestCranial neural crest (Cardiac neural crest complex) · Truncal neural crestRostral neuropore
Cephalic flexure · Pontine flexure
Alar plate (sensory) · Basal plate (motor)
Germinal matrixEye development Auditory development M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
M: EAR
anat(e/p)/phys/devp
noco/cong, epon
proc, drug(S2)
Human cell types / list derived primarily from ectoderm Surface ectoderm Trichocyte · KeratinocyteNeural crest glia: Schwann cell · Satellite glial cellDigestive systemNeural tube Categories:- Embryology of nervous system
Wikimedia Foundation. 2010.