- Chlorodifluoromethane
-
Chlorodifluoromethane 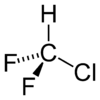
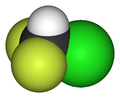 ChlorodifluoromethaneOther namesDifluoromonochloromethane, Monochlorodifliuoromethane, HCFC-22, R-22, Genetron 22, Freon 22, Arcton 4, Arcton 22, UN 1018,
ChlorodifluoromethaneOther namesDifluoromonochloromethane, Monochlorodifliuoromethane, HCFC-22, R-22, Genetron 22, Freon 22, Arcton 4, Arcton 22, UN 1018,Identifiers CAS number 75-45-6 
PubChem 6372 ChemSpider 6132 
EC number 200-871-9 KEGG D03789 
ChEMBL CHEMBL116155 
RTECS number PA6390000 Jmol-3D images Image 1 - ClC(F)F
Properties Molecular formula CHClF2 Molar mass 86.47 g/mol Appearance Colorless gas Density 3.66 kg/m3 at 15°C, gas Melting point -175.42 °C, 98 K, -284 °F
Boiling point -40.7 °C, 232 K, -41 °F
Solubility in water 0.7799 vol/vol at 25 °C; 3.628 g/L log P 1.08 Vapor pressure 908 kPa at 20 °C kH 0.033 mol.kg-1.bar-1 Structure Molecular shape Tetrahedral Hazards R-phrases R59 S-phrases S23 S24 S25 S59 Main hazards Dangerous for the environment (N), Central nervous system depressant, Carc. Cat. 3 NFPA 704 Autoignition
temperature632 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications. These applications are being phased out due to ozone depletion potential and status as a potent greenhouse gas, with a high global warming potential. R22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.
Contents
Production and current applications
Chlorodifluoromethane is prepared from chloroform:
- HCCl3 + 2 HF → HCF2Cl + 2 HCl
The main application of R22 is as a precursor to tetrafluoroethylene. This conversion involves pyrolysis to give difluorocarbene, which dimerizes:[1]
- 2 CHClF2 → C2F4 + 2 HCl
The compound also yields difluorocarbene upon treatment with strong base and is used in the laboratory as a source of this reactive intermediate.
The pyrolysis of R22 in the presence of chlorofluoromethane gives hexafluorobenzene.
Environmental effects
Chlorodifluoromethane was used as an alternative to the highly ozone-depleting CFC-11 and CFC-12, because of its relatively low ozone depletion potential of 0.055,[2] among the lowest for chlorine-containing haloalkanes. However, even this lower ozone depletion potential is no longer considered acceptable.
As an additional environmental concern, chlorodifluoromethane has a global warming potential that is 1810 (1810 times that of carbon dioxide).[3] HFCs such as R-410A have high global warming potential, but has an ODP (or ozone depletion potential) of 0. The GWP of propane (R-290), for example, is only 3.
EPA Phaseout
It will be phased out soon under the Montreal Protocol, to be replaced by other refrigerants with lower ozone depletion potential such as propane (R-290), R-410A (an azeotropic mixture of difluoromethane and pentafluoroethane), R-507A, R-134a (1,1,1,2-tetrafluoroethane) and R-409A. [4]
- Beginning January 1, 2004: The Montreal Protocol required the U.S. to reduce its consumption of HCFCs by 35% below the U.S. baseline cap. As of January 1, 2003, EPA banned production and import of HCFC-141b, the most ozone-destructive HCFC. This action allowed the United States to meet its obligations under the Montreal Protocol. EPA was able to issue 100% of company baseline allowances for production and import of HCFC-22 and HCFC-142b.
- Beginning January 1, 2010: The Montreal Protocol requires the U.S. to reduce its consumption of HCFCs by 75% below the U.S. baseline. Allowance holders may only produce or import HCFC-22 to service existing equipment. Virgin R-22 may not be used in new equipment. As a result, heating, ventilation and air-conditioning (HVAC) system manufacturers may not produce new air conditioners and heat pumps containing R-22.
- Beginning January 1, 2015: The Montreal Protocol requires the U.S. to reduce its consumption of HCFCs by 90% below the U.S. baseline.
- Beginning January 1, 2020: The Montreal Protocol requires the U.S. to reduce its consumption of HCFCs by 99.5% below the U.S. baseline. Refrigerant that has been recovered and recycled/reclaimed will be allowed beyond 2020 to service existing systems, but chemical manufacturers will no longer be able to produce R-22 to service existing air conditioners and heat pumps.
Beginning in 2010 in the U.S., the production and importing of HCFC-22 will be limited to 25% of each country's 1989 consumption level. New and imported HCFC-22 will be available only for use in equipment manufactured before 1/1/2010.
On January 1, 2010, it became illegal to import, produce, or sell R-22 for use in new equipment or pre-charged in new equipment. In 2015, the production and importing of HCFC-22 will be limited to 10% of each country's 1989 consumption level and in 2020, production and importing of HCFC-22 will be illegal. Re-use of recovered HCFC-22 to service existing equipment will be allowed indefinitely.
Physical Properties
Property Value Density (ρ) at -69 °C (liquid) 1.49 g.cm−3 Density (ρ) at -41 °C (liquid) 1.413 g.cm−3 Density (ρ) at -41 °C (gas) 4.706 kg.m−3 Density (ρ) at 15 °C (gas) 3.66 kg.m−3 Specific gravity at 21 °C (gas) 3.08 (air = 1) Specific volume (ν) at 21 °C (gas) 0.275 m³.kg−1 Density (ρ) at 15 °C (gas) 3.66 kg.m−3 Triple point temperature (Tt) -157.39 °C (115.76 K) Critical temperature (Tc) 96.2 °C (369.3 K) Critical pressure (pc) 4.936 MPa (49.36 bar) Critical density (ρc) 6.1 mol.l−1 Latent heat of vaporization (lv) at boiling point (-40.7 °C) 233.95 kJ.kg−1 Heat capacity at constant pressure (Cp) at 30 °C (86 °F) 0.057 kJ.mol−1.K−1 Heat capacity at constant volume (Cv) at 30 °C (86 °F) 0.048 kJ.mol−1.K−1 Heat capacity ratio (γ) at 30 °C (86 °F) 1.178253 Compressibility factor (Z) at 15 °C 0.9831 Acentric factor (ω) 0.22082 Molecular dipole moment 1.458 D Viscosity (η) at 0 °C 12.56 µPa.s (0.1256 cP) Ozone depletion potential (ODP) 0.055 (CCl3F = 1) Global warming potential (GWP) 1810 (CO2 = 1) It has two allotropes: crystalline II below 59 K and crystalline I above 59 K to 115.73 K.
External links
- MSDS at Oxford University
- International Chemical Safety Card 0049
- Data at Integrated Risk Information System: IRIS 0657
- Phase change data at webbook.nist.gov
- IR absorption spectra
- IARC Summaries & Evaluations: Vol. 41 (1986), Suppl. 7 (1987), Vol. 71 (1999)
References
- ^ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick "Fluorine Compounds, Organic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_349
- ^ The Montreal Protocol on Substances that Deplete the Ozone Layer. UNEP, 2000. ISBN 92-807-1888-6
- ^ IPCC (2007), Changes in Atmospheric Constituentsand in Radiative Forcing, http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf
- ^ http://www.epa.gov/ozone/title6/phaseout/22phaseout.html EPA Phase-out
Halomethanes Monosubstituted Disubstituted Trisubstituted Categories:- Halomethanes
- Hydrochlorofluorocarbons
- Ozone depletion
- Refrigerants
- Propellants
- Airsoft
- IARC Group 3 carcinogens
Wikimedia Foundation. 2010.

