- Progestogen-only pill
-
Progestogen-Only Pill (POP) Background Birth control type Hormonal First use 1973 Failure rates (first year) Perfect use 0.5% Typical use 8.0% Usage Duration effect 1 day Reversibility Yes User reminders Taken within same 3-hour window each day Clinic review 6 months Advantages and disadvantages STD protection No Weight No proven effect Periods Light spotting may be irregular Periods Often lighter and less painful Medical notes Unaffected by being on most (but not all) antibiotics. May be used, unlike COCPs, in patients with hypertension and history of migraines. Affected by some anti-epileptics. Progestogen-Only Pills or Progestin-Only Pills (POP) are contraceptive pills that contain only synthetic progestogens (progestins) and do not contain estrogen. They are colloquially known as mini pills.
Although such pills are sometimes called "Progesterone-Only Pills", they do not actually contain progesterone, but one of several chemically related compounds; and there are a number of progestogen only contraceptive formulations.
Contents
Mechanism of action
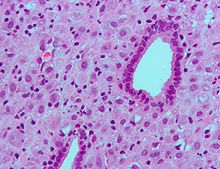 Micrograph showing decidualization of the endometrium (as also seen in pregnancy) due to (exogenous) progesterone. H&E stain.
Micrograph showing decidualization of the endometrium (as also seen in pregnancy) due to (exogenous) progesterone. H&E stain.
The mechanism of action of progestogen-only contraceptives depends on the progestogen activity and dose.[1]
- Very-low-dose progestogen-only contraceptives, such as traditional progestogen-only pills (and subdermal implants Norplant and Jadelle and intrauterine systems Progestasert and Mirena), inconsistently inhibit ovulation in ~50% of cycles and rely mainly on their progestogenic effect of thickening the cervical mucus, thereby reducing sperm viability and penetration.
- Intermediate-dose progestogen-only contraceptives, such as the progestogen-only pill Cerazette (or the subdermal implant Implanon), allow some follicular development (part of the steps of ovulation) but much more consistently inhibit ovulation in 97–99% of cycles. The same cervical mucus changes occur as with very-low-dose progestogens.
- High-dose progestogen-only contraceptives, such as the injectables Depo-Provera and Noristerat, completely inhibit follicular development (see above) and ovulation. The same cervical mucus changes occur as with very-low-dose and intermediate-dose progestogens.
In anovulatory cycles using progestogen-only contraceptives, the endometrium is thin and atrophic. If the endometrium were also thin and atrophic during an ovulatory cycle, this could, in theory, interfere with implantation of a blastocyst (embryo).
Efficacy and effectiveness
The theoretical efficacy is similar to that of the combined oral contraceptive pill (COCP). However, this pill is taken continuously without any breaks between packets, and traditional progestogen-only pills must be taken to a much stricter time every day (within 3 hours vs. a COCP's 12 hours. However, in some countries, the POP Cerazette has an approved window of 12 hours). The effectiveness is, therefore, dependent upon compliance.
POPs are not dependent upon gut bacterial flora for their absorption and so are not affected by courses of antibiotics. They will, however, be affected by any episodes of diarrhea or vomiting.
Benefits
Lacking the estrogen of combined pills, they are not associated with increased risks of DVT or heart disease. With the decreased clotting risk, they are not contraindicated in the setting of sickle-cell disease. The progestin-only pill is recommended over regular birth control pills for women who are breastfeeding because the mini-pill does not affect milk production (estrogen reduces the amount of breast milk). Like combined pills, the minipill decreases the likelihood of pelvic inflammatory disease.
It is unclear whether POPs provide protection against endometrial cancer and ovarian cancer to the extent that COCP do.
Side-effects
- With no break in the dosage, menstrual flow does not initially occur at a predictable time. Most women tend to establish, over a few months, light spotting at approximately regular intervals.
- May cause mastalgia (breast tenderness) or mood swings.
- Weight gain is less commonly experienced than on COCP.
- Some women may experience abdominal cramps and heavy bleeding.
There are fewer serious complications than on COCP.[2]
Breast cancer risk
Epidemiological evidence on POPs and breast cancer risk is based on much smaller populations of users and so is less conclusive than that for COCPs.
In the largest (1996) reanalysis of previous studies of hormonal contraceptives and breast cancer risk, less than 1% were POP users. Current or recent POP users had a slightly increased relative risk (RR 1.17) of breast cancer diagnosis that just missed being statistically significant. The relative risk was similar to that found for current or recent COCP users (RR 1.16), and, as with COCPs, the increased relative risk decreased over time after stopping, vanished after 10 years, and was consistent with being due to earlier diagnosis or promoting the growth of a preexisting cancer.[3][4]
The most recent (1999) IARC evaluation of progestogen-only hormonal contraceptives reviewed the 1996 reanalysis as well as 4 case-control studies of POP users included in the reanalysis. They concluded that: "Overall, there was no evidence of an increased risk of breast cancer" with progestogen-only contraceptives, but since there was "inadequate evidence", they were "possibly carcinogenic".[5]
Recent anxieties about the contribution of progestogens to the increased risk of breast cancer associated with HRT in postmenopausal women such as found in the WHI trials[6] have not spread to progestogen-only contraceptive use in premenopausal women.[1]
See also
References
- ^ a b Glasier, Anna (2006). "Contraception". In in DeGroot, Leslie J.; Jameson, J. Larry (eds.). Endocrinology (5th ed.). Philadelphia: Elsevier Saunders. pp. 2993–3003. ISBN 0-7216-0376-9.
- ^ Author: Laura Borgelt-Hansen.Oral Contraceptives: An Update on Health Benefits and Risks: Progestin-Only Minipill J Am Pharm Assoc. 2001;41(6)
- ^ Collaborative Group on Hormonal Factors in Breast Cancer (1996). "Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies". Lancet 347 (9017): 1713–27. doi:10.1016/S0140-6736(96)90806-5. PMID 8656904.
- ^ Collaborative Group on Hormonal Factors in Breast Cancer (1996). "Breast cancer and hormonal contraceptives: further results". Contraception 54 (3 Suppl): 1S–106S. doi:10.1016/0010-7824(96)00111-4. PMID 8899264.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (1999). "Hormonal contraceptives, progestogens only". Hormonal contraception and post-menopausal hormonal therapy; IARC monographs on the evaluation of carcinogenic risks to humans, Volume 72. Lyon: IARC Press. pp. 339–397. ISBN 92-832-1272-X. http://www.inchem.org/documents/iarc/vol72/vol72-2.html.
- ^ Chlebowski R, Hendrix S, Langer R, Stefanick M, Gass M, Lane D, Rodabough R, Gilligan M, Cyr M, Thomson C, Khandekar J, Petrovitch H, McTiernan A (2003). "Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial.". JAMA 289 (24): 3243–53. doi:10.1001/jama.289.24.3243. PMID 12824205.
Birth control methods (G02B, G03A) Comparison Behavioral Avoiding vaginal intercourse: Abstinence • Anal sex • Masturbation • Non-penetrative sex • Oral sex
Including vaginal intercourse: Breastfeeding infertility (LAM) • Calendar-based methods (rhythm, etc.) • Fertility awareness • WithdrawalBarrier or
spermicidalHormonal
(formulations)Progestogen-onlyAnti-estrogen Ormeloxifene (Centchroman)Post-intercourse Emergency contraception (pills or copper IUD) (Yuzpe regimen, Ulipristal acetate)Intrauterine device Abortion Sterilization Categories:
Wikimedia Foundation. 2010.
