- Short linear motif
-
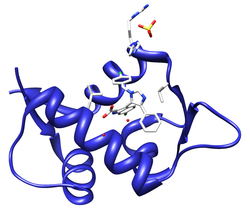
 The Human papilloma virus E7 oncoprotein mimic of the LxCxE motif (red) bound to the host Retinoblastoma protein (dark grey)(PDB 1gux)
The Human papilloma virus E7 oncoprotein mimic of the LxCxE motif (red) bound to the host Retinoblastoma protein (dark grey)(PDB 1gux)
In molecular biology Short Linear Motifs (also known as SLiMs, Linear Motifs or minimotifs) are short stretches of protein sequence that mediate protein protein interaction.[1][2]
The first definition was given by Tim Hunt:[3]
“The sequences of many proteins contain short, conserved motifs that are involved in recognition and targeting activities, often separate from other functional properties of the molecule in which they occur. These motifs are linear, in the sense that three-dimensional organization is not required to bring distant segments of the molecule together to make the recognizable unit. The conservation of these motifs varies: some are highly conserved while others, for example, allow substitutions that retain only a certain pattern of charge across the motif.”
Contents
Attributes
SLiMs are generally situated in intrinsically disordered regions (over 80% of known SLiMs), however, upon interaction with a structured partner secondary structure is often induced. The majority of annotated SLiMs consist of 3 to 11 contiguous amino acids, with an average of just over 6 residues. However, only few hotspot residues (on average 1 hotspot for each 3 residues in the motif) contribute the majority of the free energy of binding and determine most of the affinity and specificity of the interaction. Although most motifs have no positional preference, several of them are required to be localized at the protein termini in order to be functional [4][5]. The key defining attribute of SLiMs, having a limited number of residues that directly contact the binding partner, has two major consequences. First, only few or even a single mutation can result in the generation of a functional motif, with further mutations of flanking residues allowing tuning affinity and specificity. This results in SLiMs having an increased propensity to evolve convergently, which facilitates their proliferation, as is evidenced by their increased incidence in higher Eukaryotes. It has been hypothesized that this might increase and restructure the connectivity of the interactome. Second, SLiMs have relatively low affinity for their interaction partners (generally between 1 and 150 μM), which makes these interactions transient and reversible, and thus ideal to mediate dynamic processes such as cell signaling. In addition, this means that these interactions can be easily modulated by post-translational modifications that change the structural and physicochemical properties of the motif. Also, regions of high functional density can mediate molecular switching by means of overlapping motifs (e.g. the C-terminal tails of integrin beta subunits), or they can allow high avidity interactions by multiple low affinity motifs (e.g. multiple AP2-binding motifs in Eps15) [5][6][7].
Function
The molecular function of a SLiM is to deliver specific interactions with additional protein domain(s). In general, the SLiM itself serves as specific information mediator whereas the result may influence the SLiM-bearing protein as a complete entity.
Consequently, in a cellular context, this may result in different functions dependent on the actual kind of interaction domain. The common way of interaction is the bare binding of the SLiM to an interaction domain that may result in being part of a protein complex, may it be as effector or as central hub of such a complex. A subset of this are targeting SLiMs that enable the SLiM bearing protein to form complexes with cellular transporter hence being able to change cellular compartments.
In case of modifying domains the effect of SLiM recognition and interaction will be a modification of the sequence, e.g. a post translational modification (PTMs) or a sequence cleavage event. In this modified state the SLiM bearing protein may be involved in additional interactions with further downstream proteins of a pathway.Overview of SLiM functions
Protein binding motifs
deliver binding specifity with domains of interacting proteins hence resulting in being part of a protein complex, may it be as effector or as central hub. They may also be involved into the co-operative assembly of scaffolds, with a typical example being SLiMs with Proline-rich sequences that are responsible for binding of SH3 domains.
Targeting motifs
are recognized by domains of cellular transporters leading to a switch in cellular compartmentalisation. Famous examples are Nuclear localisation signals (NLSs) and Nuclear export signals (NESs) together being capable to deliver the nuclear shuttling capablities of tumor suppressor proteins in a fine-tuned fashion.
Posttranslational modifications
may result in phosphorylation, myristoylation, N-linked glycosylation or other PTMs often being part of bigger signal communication.
Cleavage sites
are recognition sites of endo-peptidases. The products may also bear specific informational content, e.g. constituting terminal-specific degrons.
Considering SLiM functions in a cellbiological perspective you would state their involvement in almost any pathway due to their critical role in protein-protein interaction and signal transduction.
Role in disease
Several diseases have been linked to mutations in SLiMs. For instance, one cause of Noonan Syndrome is a mutation in the protein Raf-1 which abrogates the interaction with 14-3-3 proteins mediated by corresponding short linear motifs and thereby deregulate the Raf-1 kinase activity [8]. Usher's Syndrome is the most frequent cause of hereditary deaf-blindness in humans [9] and can be caused by mutations in either PDZ domains in Harmonin or the corresponding PDZ interaction motifs in the SANS protein [10]. Finally, Liddle's Syndrome has been implicated with autosomal dominant activating mutations in the WW interaction motif in the β-(SCNNB_HUMA) and γ-(SCNNG_HUMA) subunits of the Epithelial_sodium_channel ENaC [11] . These mutations abrogate the binding to the ubiquitin ligase NEDD4, thereby inhibiting channel degradation and prolonging the half-life of ENaC, ultimately resulting in increased Na+ reabsorption, plasma volume extension and hypertension [12].
Viruses often mimic human SLiMs to hijack and disrupt a host's cellular machinery[13][14], thereby adding functionality to their compact genomes without necessitating new virally encoded proteins. In fact, many motifs were originally discovered in viruses, such as the Retinoblastoma binding LxCxE motif and the UEV domain binding PTAP late domain. The short generation times and high mutation rates of viruses, in association with natural selection, has led to multiple examples of mimicry of host SLiMs in every step of the viral life cycle (Src binding motif PxxP in Nef modulates replication, WW domain binding PPxY mediates budding in Ebola virus, A Dynein Light Chain binding motif in Rabies virus is vital for host infection). The extent of human SLiM mimicry is surprising with many viral proteins containing several functional SLiMs, for example, the Adenovirus protein E1A.
Pathogenic bacteria also mimic host motifs (as well as having their own motifs), however, not to the same extent as the obligate parasite viruses. E. Coli injects a protein, EspF(U), that mimics an autoinhibitory element of N-WASP into the host cell to activate actin-nucleating factors WASP [15]. The KDEL motif of the bacteriophage encoded cholera toxin mediates cell entry of the cholera bacterium [16].
Potential as leads for drug design
Linear motif mediated protein-protein interactions have shown promise in recent years as novel drug targets[17]. Success stories include the MDM2 motif analog Nutlin-3 and integrin targeting RGD-mimetic Cilengitide: Nutlin-3 antagonises the interaction of MDM2's SWIB domain with p53 thus stabilising p53 and inducing senescence in cancer cells [18]. Cilengitide inhibits integrin-dependent signaling, causing the disassembly of cytoskeleton, cellular detachment and the induction of apoptosis in endothelial and glioma cells.[19][20]. In addition, peptides targeting the Grb2 and Crk SH2/ SH3 adaptor domains are also under investigation [21][22].
There are at present no drugs on the market specially targeting phosphorylation sites, however, a number of drugs target the kinase domain. This tactic has shown promise in the treatments of various forms of cancer [23]. For example, Stutnet® is a receptor tyrosine kinase (RTK) inhibitor for treating gastrointestinal cancer, Gleevec® specially targets bcr-abl and Sprycel® is a broad-based tyrosine kinase inhibitor whose targets include Bcr-Abl and Src. Cleavage is another process directed by motif recognition with the proteases responsible for cleavage a good drug target. For example, Tritace®, Vasotec®, Accupril®, and Lotensin® are substrate mimetic Angiotensin converting enzymes inhibitors. Other drugs that target post-translational modifications include Zovirax®, an antiviral myristoylation inhibitor and Farnysyl Transferase inhibitors that block the lipidation modification to a CAAX-box motif.
Recommended further reading: [24][23]
Computational motif resources
Databases
SLiMs are usually described by regular expressions in the motif literature with the important residues defined based on a combination of experimental, structural and evolutionary evidence. However, high throughput screening such as phage display has seen a large increase in the available information for many motifs classes allowing them to be described with sequence logos. Several diverse repositories currently curate the available motif data. In terms of scope, the Eukaryotic Linear Motif resource (ELM)[25] and MiniMotif Miner (MnM) [26] represent the two largest motif databases as they attempt to capture all motifs from the available literature. Several more specific and specialised databases also exist, PepCyber[27] and ScanSite[28] focus on smaller subsets of motifs, phosphopeptide binding and important signaling domains respectively. PDZBase[29] focuses solely on PDZ domain ligands. Merops[30] and CutDB[31] curate available proteolytic event data including protease specificity and cleavage sites. There has been a large increase in the number of publications describing motif mediated interactions over past decade and as a result a large amount of the available literature remains to be curated. Recent work has created the tool MiMosa[32] to expedite the annotation process and encourage semantically robust motif descriptions[33].
Discovery tools
SLiMs are short and degenerate and as a result the proteome is littered with stochastically occurring peptides that resemble functional motifs. The biologically relevant cellular partners can easily distinguish functional motifs, however computational tools have yet to reach a level of sophistication where motif discovery can be accomplished with high success rates.
Motif discovery tools can be split into two major categories, discovery of novel instance of known functional motifs class and discovery of functional motifs class, however, they all use a limited and overlapping set of attributes to discriminate true and false positives. The main discrimatory attributes used in motif discovery are:
- Accessibility - the motif must be accessible for the binding partner. Intrinsic disorder prediction tools (such as IUPred or GlobPlot), domain databases (such as Pfam and SMART) and experimentally derived structural data (from sources such as PDB) can be used to check the accessibility of predicted motif instances.
- Conservation - the conservation of a motif correlates strongly with functionality and many experimental motifs are seen as islands of strong constraint in regions of weak conservation. Alignment of homologous proteins can be used to calculate conservation metric for a motif.
- Physicochemical properties - Certain intrinsic properties of residues or stretches of amino acids are strong discriminators of functionality, for example, the propensity of a region of disorder to undergo a disorder to order transition.
- Enrichment in groupings of similar proteins - Motif often evolve convergently to carry out similar tasks in different proteins such as mediating binding to a specific partner or targeting proteins to a particular subcellular localisation. Often in such cases these grouping the motif occurs more often than is expected by chance and can be detected by searching for enriched motifs.
Novel functional motifs instances
The Eukaryotic Linear Motif resource (ELM)[25] and MiniMotif Miner (MnM) [26] both provide servers to search for novel instance of known functional motifs in protein sequences. SLiMSearch allows similar searches on a proteome-wide scale [34].
Novel functional motifs class
More recently computational methods have been developed that can identify new Short Linear Motifs de novo. Interactome-based tools rely on identifying a set of proteins that are likely to share a common function, such as binding the same protein or being cleaved by the same peptidase. Two examples of such software are DILIMOT and SLiMFinder.[35][36]. Anchor and α-MoRF-Pred use physicochemical properties to search for motif-like peptides in disordered regions. ANCHOR [37] identifies stretches of intrinsically disordered regions that cannot form favorable intrachain interactions to fold without additional stabilising energy contributed by a globular interaction partner. α-MoRF-Pred [38] uses the inherent propensity of many SLiM to under go a disorder to order transition upon binding to discover α-helical forming stretches within disordered regions.
References
- ^ Diella F, Haslam N, Chica C, et al. (2008). "Understanding eukaryotic linear motifs and their role in cell signaling and regulation". Front. Biosci. 13: 6580–603. PMID 18508681.
- ^ Neduva V, Russell RB (October 2006). "Peptides mediating interaction networks: new leads at last". Curr. Opin. Biotechnol. 17 (5): 465–71. doi:10.1016/j.copbio.2006.08.002. PMID 16962311.
- ^ Hunt T (1990). "Protein sequence motifs involved in recognition and targeting: a new series.". Trends Biochem. Sci. 15 (8): 305–9. PMID 2204156.
- ^ London, N.; Movshovitz-Attias, D.; Schueler-Furman, O. (2010). "The Structural Basis of Peptide-Protein Binding Strategies". Structure 18 (2): 188–199. doi:10.1016/j.str.2009.11.012. PMID 20159464.
- ^ a b Davey, N. E.; Van Roey, K.; Weatheritt, R. J.; Toedt, G.; Uyar, B.; Altenberg, B.; Budd, A.; Diella, F. et al. (2012). "Attributes of short linear motifs". Molecular BioSystems. doi:10.1039/c1mb05231d. PMID 21909575.
- ^ Neduva, V.; Russell, R. (2005). "Linear motifs: Evolutionary interaction switches". FEBS Letters 579 (15): 3342–3345. doi:10.1016/j.febslet.2005.04.005. PMID 15943979.
- ^ Gibson, T. J. (2009). "Cell regulation: Determined to signal discrete cooperation". Trends in Biochemical Sciences 34 (10): 471–482. doi:10.1016/j.tibs.2009.06.007. PMID 19744855.
- ^ Pandit, B.; Sarkozy, A.; Pennacchio, L. A.; Carta, C.; Oishi, K.; Martinelli, S.; Pogna, E. A.; Schackwitz, W. et al. (2007). "Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy". Nature Genetics 39 (8): 1007–1012. doi:10.1038/ng2073. PMID 17603483.
- ^ Eudy, J. D.; Sumegi, J. (1999). "Molecular genetics of Usher syndrome". Cellular and molecular life sciences : CMLS 56 (3–4): 258–267. PMID 11212353.
- ^ Kalay, E.; Brouwer, A. P. M.; Caylan, R.; Nabuurs, S. B.; Wollnik, B.; Karaguzel, A.; Heister, J. G. A. M.; Erdol, H. et al. (2005). "A novel D458V mutation in the SANS PDZ binding motif causes atypical Usher syndrome". Journal of Molecular Medicine 83 (12): 1025–1032. doi:10.1007/s00109-005-0719-4. PMID 16283141.
- ^ Warnock, D. G. (1998). "Liddle syndrome: An autosomal dominant form of human hypertension". Kidney International 53 (1): 18–24. doi:10.1046/j.1523-1755.1998.00728.x. PMID 9452995.
- ^ Furuhashi, M.; Kitamura, K.; Adachi, M.; Miyoshi, T.; Wakida, N.; Ura, N.; Shikano, Y.; Shinshi, Y. et al. (2004). "Liddle's Syndrome Caused by a Novel Mutation in the Proline-Rich PY Motif of the Epithelial Sodium Channel -Subunit". Journal of Clinical Endocrinology & Metabolism 90 (1): 340–344. doi:10.1210/jc.2004-1027. PMID 15483078.
- ^ Davey NE, Travé G, Gibson TJ (March 2011). "How viruses hijack cell regulation". Trends Biochem. Sci. 36 (3): 159–69. doi:10.1016/j.tibs.2010.10.002. PMID 21146412.
- ^ Kadaveru K, Vyas J, Schiller MR (2008). "Viral infection and human disease--insights from minimotifs". Front. Biosci. 13: 6455–71. PMC 2628544. PMID 18508672. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2628544.
- ^ Sallee, N. A.; Rivera, G. M.; Dueber, J. E.; Vasilescu, D.; Mullins, R. D.; Mayer, B. J.; Lim, W. A. (2008). "The Pathogen Protein EspFU Hijacks Actin Polymerization Using Mimicry and Multivalency". Nature 454 (7207): 1005–1008. doi:10.1038/nature07170. PMC 2749708. PMID 18650806. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2749708.
- ^ Lencer, W. I.; Constable, C.; Moe, S.; Jobling, M. G.; Webb, H. M.; Ruston, S.; Madara, J. L.; Hirst, T. R. et al. (1995). "Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: Role of COOH-terminal KDEL". The Journal of cell biology 131 (4): 951–962. PMC 2200010. PMID 7490296. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2200010.
- ^ Wells JA, McClendon CL (2007). "Reaching for high-hanging fruit in drug discovery at protein-protein interfaces". Nature: 1001–9. PMID 18075579.
- ^ Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. (2004). "In vivo activation of the p53 pathway by small-molecule antagonists of MDM2.". Science: 844–8. PMID 14704432.
- ^ Goodman SL, Hölzemann G, Sulyok GA, Kessler H. (2002). "Nanomolar small molecule inhibitors for alphav(beta)6, alphav(beta)5, and alphav(beta)3 integrins.". J Med Chem: 1045–51. PMID 11855984.
- ^ Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I, Schuch G. (2008). "Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway.". J Exp Clin Cancer Res: 86. PMID 19114005.
- ^ Gril B, Vidal M, Assayag F, Poupon MF, Liu WQ, Garbay C. (2007). "Grb2-SH3 ligand inhibits the growth of HER2+ cancer cells and has antitumor effects in human cancer xenografts alone and in combination with docetaxel.". Int J Cancer..: 407–15. PMID 17372910.
- ^ Feller SM, Lewitzky M. (2006). "Potential disease targets for drugs that disrupt protein-- protein interactions of Grb2 and Crk family adaptors.". Curr Pharm Des.: 529–48. PMID 16472145.
- ^ a b Kadaveru K, Vyas J, Schiller MR. (2008). "Viral infection and human disease--insights from minimotifs.". Front Biosci.: 6455–71. PMC 2628544. PMID 18508672. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2628544.
- ^ Metallo SJ. (2010). "Intrinsically disordered proteins are potential drug targets.". FCurr Opin Chem Biol.: 481–8. doi:10.1016/j.cbpa.2010.06.169. PMID 20598937.
- ^ a b Gould CM, Diella F, Via A, et al. (January 2010). "ELM: the status of the 2010 eukaryotic linear motif resource". Nucleic Acids Res. 38 (Database issue): D167–80. doi:10.1093/nar/gkp1016. PMC 2808914. PMID 19920119. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2808914.
- ^ a b Rajasekaran S, Balla S, Gradie P, et al. (January 2009). "Minimotif miner 2nd release: a database and web system for motif search". Nucleic Acids Res. 37 (Database issue): D185–90. doi:10.1093/nar/gkn865. PMC 2686579. PMID 18978024. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2686579.
- ^ Gong, W.; Zhou, D.; Ren, Y.; Wang, Y.; Zuo, Z.; Shen, Y.; Xiao, F.; Zhu, Q. et al. (2007). "PepCyber:P∼PEP: A database of human protein–protein interactions mediated by phosphoprotein-binding domains". Nucleic Acids Research 36 (Database issue): D679–D683. doi:10.1093/nar/gkm854. PMC 2238930. PMID 18160410. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2238930.
- ^ Obenauer, J. C.; Cantley, L. C.; Yaffe, M. B. (2003). "Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs". Nucleic acids research 31 (13): 3635–3641. PMC 168990. PMID 12824383. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=168990.
- ^ Beuming, T.; Skrabanek, L.; Niv, M. Y.; Mukherjee, P.; Weinstein, H. (2004). "PDZBase: A protein-protein interaction database for PDZ-domains". Bioinformatics 21 (6): 827–828. doi:10.1093/bioinformatics/bti098. PMID 15513994.
- ^ Rawlings, N. D.; Barrett, A. J.; Bateman, A. (2009). "MEROPS: The peptidase database". Nucleic Acids Research 38 (Database issue): D227–D233. doi:10.1093/nar/gkp971. PMC 2808883. PMID 19892822. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2808883.
- ^ Igarashi, Y.; Eroshkin, A.; Gramatikova, S.; Gramatikoff, K.; Zhang, Y.; Smith, J. W.; Osterman, A. L.; Godzik, A. (2007). "CutDB: A proteolytic event database". Nucleic Acids Research 35 (Database issue): D546–D549. doi:10.1093/nar/gkl813. PMC 1669773. PMID 17142225. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1669773.
- ^ Vyas, J.; Nowling, R. J.; Meusburger, T.; Sargeant, D.; Kadaveru, K.; Gryk, M. R.; Kundeti, V.; Rajasekaran, S. et al. (2010). "MimoSA: A system for minimotif annotation". BMC Bioinformatics 11: 328. doi:10.1186/1471-2105-11-328. PMC 2905367. PMID 20565705. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2905367.
- ^ Praefcke GJ, Ford MG, Schmid EM, et al. (November 2004). "A Syntax For Minimotifs, Version 1". BMC Genomics 23 (22): 4371–83. doi:10.1038/sj.emboj.7600445. PMC 526462. PMID 15496985. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=526462.
- ^ Davey, N. E.; Haslam, N. J.; Shields, D. C.; Edwards, R. J. (2011). "SLiMSearch 2.0: Biological context for short linear motifs in proteins". Nucleic Acids Research 39 (Web Server issue): W56–W60. doi:10.1093/nar/gkr402. PMC 3125787. PMID 21622654. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3125787.
- ^ Neduva V, Russell RB (July 2006). "DILIMOT: discovery of linear motifs in proteins". Nucleic Acids Res. 34 (Web Server issue): W350–5. doi:10.1093/nar/gkl159. PMC 1538856. PMID 16845024. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1538856.
- ^ Davey NE, Haslam NJ, Shields DC, Edwards RJ (2010). "SLiMFinder: a web server to find novel, significantly over-represented, short protein motifs.". Nucleic Acids Res. 38 (Webserver Issue): W534-9. Epub. doi:10.1093/nar/gkq440. PMC 2896084. PMID 20497999. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2896084.
- ^ Mészáros, B. L.; Simon, I. N.; Dosztányi, Z. (2009). Casadio, Rita. ed. "Prediction of Protein Binding Regions in Disordered Proteins". PLoS Computational Biology 5 (5): e1000376. doi:10.1371/journal.pcbi.1000376. PMC 2671142. PMID 19412530. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2671142.
- ^ Cheng, Y.; Oldfield, C. J.; Meng, J.; Romero, P.; Uversky, V. N.; Dunker, A. K. (2007). "Mining α-helix-forming molecular recognition features (α-MoRFs) with cross species sequence alignments". Biochemistry 46 (47): 13468–13477. doi:10.1021/bi7012273. PMC 2570644. PMID 17973494. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2570644.
External links
SLiM databases
SLiM discovery tools
- ANCHOR
- Eukaryotic Linear Motif Database
- MiniMotif Miner
- SLiMSuite : Website for SLiMFinder, SLiMSearch and CompariMotif
- ScanSite
Categories:- Biology
- Protein structural motifs
- Protein domains
Wikimedia Foundation. 2010.