- Leishmaniasis
-
Leishmaniasis Classification and external resources 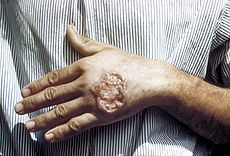
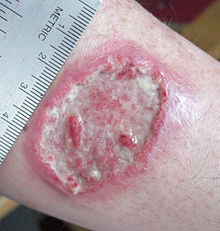
Cutaneous leishmaniasis in the hand of a Central American adultICD-10 B55 ICD-9 085 DiseasesDB 3266 29171 3266 7070 MedlinePlus 001386 eMedicine emerg/296 MeSH D007896 Leishmaniasis is a disease caused by protozoan parasites that belong to the genus Leishmania and is transmitted by the bite of certain species of sand fly (subfamily Phlebotominae). Although the majority of the literature mentions only one genus transmitting Leishmania to humans (Lutzomyia) in the Americas, a 2003 study by Galati suggested a new classification for the New World sand flies, elevating several subgenera to the genus level. Elsewhere in the world, the genus Phlebotomus is considered the vector of leishmaniasis.[1]
Most forms of the disease are transmissible only from animals (zoonosis), but some can be spread between humans. Human infection is caused by about 21 of 30 species that infect mammals. These include the L. donovani complex with three species (L. donovani, L. infantum, and L. chagasi); the L. mexicana complex with four main species (L. mexicana, L. amazonensis, and L. venezuelensis); L. tropica; L. major; L. aethiopica; and the subgenus Viannia with four main species (L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) panamensis, and L. (V.) peruviana). The different species are morphologically indistinguishable, but they can be differentiated by isoenzyme analysis, DNA sequence analysis, or monoclonal antibodies.
Cutaneous leishmaniasis is the most common form of leishmaniasis. Visceral leishmaniasis is a severe form in which the parasites have migrated to the vital organs.
Contents
Classification
Leishmaniasis may be divided into the following types:[2]:422–428
- Cutaneous leishmaniasis
- Mucocutaneous leishmaniasis
- Visceral leishmaniasis
- Post-kala-azar dermal leishmaniasis
- Viscerotropic leishmaniasis
Signs and symptoms
The symptoms of leishmaniasis are skin sores which erupt weeks to months after the person affected is bitten by sand flies. Other consequences, which can manifest anywhere from a few months to years after infection, include fever, damage to the spleen and liver, and anemia.
In clinical medicine, leishmaniasis is considered one of the classic causes of a markedly enlarged (and therefore palpable) spleen; the organ, which is not normally felt during examination of the abdomen, may become larger even than the liver in severe cases.
There are four main forms of leishmaniasis:
- Visceral leishmaniasis – the most serious form and potentially fatal if untreated.
- Cutaneous leishmaniasis – the most common form which causes a sore at the bite site, which heals in a few months to a year, leaving an unpleasant looking scar. This form can progress to any of the other three forms.
- Diffuse cutaneous leishmaniasis – this form produces widespread skin lesions which resemble leprosy and is particularly difficult to treat.
- Mucocutaneous leishmaniasis – commences with skin ulcers which spread causing tissue damage, to, (particularly), the nose and mouth.
Mechanism
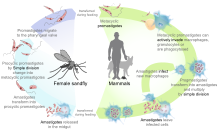
Leishmaniasis is transmitted by the bite of female phlebotomine sandflies. The sandflies inject the infective stage, metacyclic promastigotes, during blood meals (1). Metacyclic promastigotes that reach the puncture wound are phagocytized by macrophages (2) and transform into amastigotes (3). Amastigotes multiply in infected cells and affect different tissues, depending in part on which Leishmania species is involved (4). These differing tissue specificities cause the differing clinical manifestations of the various forms of leishmaniasis. Sandflies become infected during blood meals on an infected host when they ingest macrophages infected with amastigotes (5,6). In the sandfly's midgut, the parasites differentiate into promastigotes (7), which multiply, differentiate into metacyclic promastigotes and migrate to the proboscis (8).
Leishmaniasis is caused by infection with the pathogen Leishmania. The genomes of three Leishmania species (L. major, L. infantum and L. braziliensis) have been sequenced and this has provided much information about the biology of the parasite. For example it is now understood that in Leishmania protein-coding genes are organized as large polycistronic units in a head-to-head or tail-to-tail manner; RNA polymerase II transcribes long polycistronic messages in the absence of defined RNA pol II promoters; and Leishmania has unique features with respect to the regulation of gene expression in response to changes in the environment. The new knowledge from these studies may help identify new targets for urgently needed drugs, and aid the development of vaccines.[1]
Diagnosis
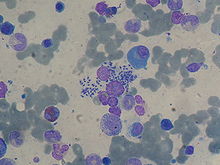
Leishmaniasis is diagnosed in the haematology laboratory by direct visualization of the amastigotes (Leishman-Donovan bodies). Buffy-coat preparations of peripheral blood or aspirates from marrow, spleen, lymph nodes or skin lesions should be spread on a slide to make a thin smear, and stained with Leishman's or Giemsa's stain (pH 7.2) for 20 minutes. Amastigotes are seen with monocytes or, less commonly in neutrophil in peripheral blood and in macrophages in aspirates. They are small, round bodies 2-4μm in diameter with indistinct cytoplasm, a nucleus and a small rod-shaped kinetoplast. Occasionally amastigotes may be seen lying free between cells.[3]
Prevention
Currently there are no vaccines in routine use. However, the genomic sequence of Leishmania has provided a rich source of vaccine candidates. Genome-based approaches have been used to screen for novel vaccine candidates. One study screened 100 randomly selected genes as DNA vaccines against L. major infection in mice. Fourteen reproducibly protective novel vaccine candidates were identified. A separate study used a two-step procedure to identify T cell antigens. Six unique clones were identified: glutamine synthetase, a transitional endoplasmic reticulum ATPase, elongation factor 1gamma, kinesin K-39, repetitive protein A2, and a hypothetical conserved protein. The 20 antigens identified in these two studies are being further evaluated for vaccine development.[4]
Treatment
There are two common therapies containing antimony (known as pentavalent antimonials), meglumine antimoniate (Glucantime) and sodium stibogluconate (Pentostam). It is not completely understood how these drugs act against the parasite; they may disrupt its energy production or trypanothione metabolism. Unfortunately, in many parts of the world, the parasite has become resistant to antimony when treating for visceral or mucocutaneous leishmaniasis,[5] but the level of resistance varies according to species.[6] Amphotericin (AmBisome) is now the treatment of choice;[7] its failure in some cases to treat visceral leishmaniasis (Leishmania donovani) has been reported in Sudan, but this may be related to host factors such as co-infection with HIV or tuberculosis rather than parasite resistance.[8]
Miltefosine (Impavido), is a new drug for visceral and cutaneous leishmaniasis. The cure rate of miltefosine in phase III clinical trials is 95%; Studies in Ethiopia show that it is also effective in Africa. In an observational study of 34 Dutch soldiers with Leishmania major infection that had failed to respond to intralesional antimony, 30 responded to miltefosine.[9] In HIV immunosuppressed people who are coinfected with leishmaniasis it has shown that even in resistant cases 2/3 of the people responded to this new treatment. Clinical trials in Colombia showed a high efficacy for cutaneous leishmaniasis. In mucocutaneous cases caused by L.brasiliensis it has shown to be more effective than other drugs. Miltefosine received approval by the Indian regulatory authorities in 2002 and in Germany in 2004. In 2005 it received the first approval for cutaneous leishmaniasis in Colombia. Miltefosine is also currently being investigated as treatment for mucocutaneous leishmaniasis caused by Leishmania braziliensis in Colombia,[5] and preliminary results are very promising. It is now registered in many countries and is the first orally administered breakthrough therapy for visceral and cutaneous leishmaniasis.[10][11] In October 2006 it received orphan drug status from the US Food and Drug administration. The drug is generally better tolerated than other drugs. Main side effects are gastrointestinal disturbance in the 1–2 days of treatment which does not affect the efficacy. Because it is available as an oral formulation, the expense and inconvenience of hospitalisation is avoided, which makes it an attractive alternative.
The Institute for OneWorld Health has reintroduced the drug paromomycin for treatment of leishmaniasis, results with which led to its approval as an orphan drug. The Drugs for Neglected Diseases Initiative is also actively facilitating the search for novel therapeutics. A treatment with paromomycin will cost about $10. The drug had originally been identified in 1960s, but had been abandoned because it would not be profitable, as the disease mostly affects poor people.[12] The Indian government approved paromomycin for sale in August 2006.[13] A 21-day course of paromomycin produces a definitive cure in >90% of patients with visceral leishmaniasis.[14]
Drug-resistant leishmaniasis may respond to immunotherapy (inoculation with parasite antigens plus an adjuvant) which aims to stimulate the body's own immune system to kill the parasite.[15]
Two weeks of topical treatment with 0.1% cantharidin ointment was an effective method for treating cutaneous leishmaniasis in infected BALB/c mice.[16]
Epidemiology
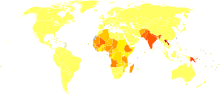 Disability-adjusted life year for leishmaniasis per 100,000 inhabitants.
Disability-adjusted life year for leishmaniasis per 100,000 inhabitants. no dataless than 2020-3030-4040-5050-6060-7070-8080-100100-120120-150150-200more than 200
no dataless than 2020-3030-4040-5050-6060-7070-8080-100100-120120-150150-200more than 200Leishmaniasis can be transmitted in many tropical and sub-tropical countries, and is found in parts of about 88 countries. Approximately 350 million people live in these areas. The settings in which leishmaniasis is found range from rainforests in Central and South America to deserts in West Asia and the Middle East. It affects as many as 12 million people worldwide, with 1.5–2 million new cases each year.[17] The visceral form of leishmaniasis has an estimated incidence of 500,000 new cases and 60,000 deaths each year.[18] More than 90 percent of the world's cases of visceral leishmaniasis are in India, Bangladesh, Nepal, Sudan, and Brazil.[19]
Leishmaniasis is found through much of the Americas from northern Argentina to southern Texas, though not in Uruguay or Chile, and has recently been shown to be spreading to North Texas.[20] Leishmaniasis is also known as papalomoyo / papa lo moyo and ulcero de los chicleros in Latin America.[21] During 2004, it is calculated that some 3,400 troops from the Colombian army, operating in the jungles near the south of the country (in particular around the Meta and Guaviare departments), were infected with leishmaniasis. Apparently, a contributing factor was that many of the affected soldiers did not use the officially provided insect repellent, because of its allegedly disturbing odor. It is estimated that nearly 13,000 cases of the disease were recorded in all of Colombia throughout 2004, and about 360 new instances of the disease among soldiers had been reported in February 2005.[22][23][24]
The disease is found across much of Asia, though not Southeast Asia, and in the Middle East. Within Afghanistan, leishmaniasis occurs commonly in Kabul, partly due to bad sanitation and waste left uncollected in streets, allowing parasite-spreading sand flies an environment they find favorable.[25][26] In Kabul the number of people infected was estimated at least 200,000, and in three other towns (Herat, Kandahar and Mazar-i-Sharif) there were about 70,000 more, according to WHO figures from 2002.[27][verification needed] Kabul is estimated as the largest center of cutaneous leishmaniasis in the world, with approximately 67,500 cases as of 2004.[28]
Africa, in particular the East and North, is home to cases of Leishamaniasis. The disease is spreading to Southern Europe but is not found in Australia or Oceania.[citation needed]
Leishmaniasis is mostly a disease of the Developing World, and is rarely known in the developed world outside a small number of cases, mostly in instances where troops are stationed away from their home countries. Leishmaniasis has been reported by U.S. troops stationed in Saudi Arabia and Iraq since the Gulf War of 1990, including visceral leishmaniasis.[29][30][31] In September 2005 the disease was contracted by at least four Dutch marines who were stationed in Mazari Sharif, Afghanistan, and subsequently repatriated for treatment.[citation needed]
History
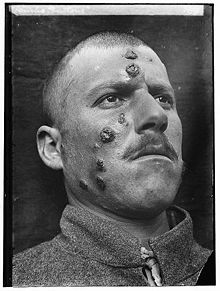 A 1917 case of cutaneous leishmaniasis in the Middle East, known then locally as "Jericho Buttons" for the frequency of cases near the ancient city of Jericho
A 1917 case of cutaneous leishmaniasis in the Middle East, known then locally as "Jericho Buttons" for the frequency of cases near the ancient city of Jericho
Descriptions of conspicuous lesions similar to cutaneous leishmaniasis (CL) has been discovered on tablets from King Ashurbanipal from the 7th century BC, some of which may have been derived from even earlier texts from 1500 to 2500 BC. Muslim physicians including Avicenna in the 10th century AD gave detailed descriptions of what was called Balkh sore.[32] In 1756, Alexander Russell, after examining a Turkish patient, gave one of the most detailed clinical descriptions of the disease. Physicians in the Indian subcontinent would describe it as Kala-azar (pronounced kālā āzār, the Urdu, Hindi and Hindustani phrase for black fever, kālā meaning black and āzār meaning fever or disease). As for the new world, evidence of the cutaneous form of the disease was found in Ecuador and Peru in pre-Inca potteries depicting skin lesions and deformed faces dating back to the first century AD. 15th and 16th century texts from the Inca period and from Spanish colonials mention "valley sickness", "Andean sickness", or "white leprosy", which are likely to be CL.[33] Who first discovered the organism is somewhat disputed. It is possible that Surgeon major Cunningham of the British Indian army saw it first in 1885 without being able to relate it to the disease.[34][35] Peter Borovsky, a Russian military surgeon working in Tashkent, conducted research into the etiology of oriental sore, locally known as Sart sore, and in 1898 published the first accurate description of the causative agent, correctly described the parasite's relation to host tissues and correctly referred it to Protozoa. However, because his results were published in Russian in a journal with low circulation, his priority was not internationally acknowledged during his lifetime.[36] In 1901, Leishman identified certain organisms in smears taken from the spleen of a patient who had died from "dum-dum fever" (Dum Dum is an area close to Calcutta) and proposed them to be trypanosomes, found for the first time in India.[37] A few months later Captain Charles Donovan (1863–1951) confirmed the finding of what became known as Leishman-Donovan bodies in smears taken from patients in Madras, India.[38] But it was Ronald Ross who proposed that Leishman-Donovan bodies were the intracellular stages of a new parasite, which he named Leishmania donovani.[39] The link with the disease kala-azar was first suggested by Charles Donovan, but was conclusively demonstrated by Charles Bentley's discovery of Leishmania donovani in patients with kala-azar.[40] The disease was a major problem for Allied troops fighting in Sicily during the Second World War, and it was then that research by Leonard Goodwin showed that pentostam was an effective treatment.[41]
Research
 A parasitologist working on L. major in a biocontainment hood
A parasitologist working on L. major in a biocontainment hood
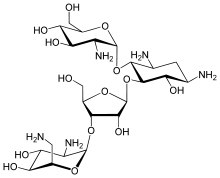
Several potential vaccines are being developed, under pressure from the World Health Organization, but as of 2010[update] none are available.[42] The team at the Laboratory for Organic Chemistry at the Swiss Federal Institute of Technology (ETH) in Zürich are trying to design a carbohydrate-based vaccine.[18] The genome of the parasite Leishmania major has been sequenced,[43] possibly allowing for identification of proteins that are used by the pathogen but not by humans; these proteins are potential targets for drug treatments.
In 2009, the Hebrew University of Jerusalem Kuvin Center for the Study of Infectious and Tropical Diseases, in a collaborative effort with Addis Ababa University, was awarded a grant by the Bill & Melinda Gates Foundation for research into visceral leishmaniasis in Ethiopia. The project will gather data to be analyzed to identify the weak links in the transmission cycle and devise methods for control of the disease.[44]
HIV protease inhibitors have been found to be active against Leishmania species in two in vitro studies in Canada and India. The study reported that the intracellular growth of Leishmania parasites was controlled by nelfinavir and ritonavir in a human monocyte cell line and also in human primary monocyte-derived macrophages.[45]
Notable cases
While filming the latest series of Extreme Dreams in Peru, top UK television presenter Ben Fogle caught the disease. He was left bedridden for three weeks on his return home. Fogle was treated at London's Hospital for Tropical Diseases.[46]
See also
- Visceral leishmaniasis (kala azar)
- Cutaneous leishmaniasis
- Canine leishmaniasis
- Leishmania
- List of parasites (human)
- Tropical disease
- CVBD Canine Vector-borne diseases
References
- ^ a b Myler P; Fasel Nhirf;f). (2008). Leishmania: After The Genome. Caister Academic Press. p. [page needed]. ISBN [[Special:BookSources/978-1-904455-28-8]|978-1-904455-28-8]]]. [http://www.horizonpress.com/leish. http://www.horizonpress.com/leish.
- ^ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. pp. [page needed]. ISBN 0-7216-2921-0.
- ^ Dacie, John V.; Bain, Barbara J.; Imelda Bates (2006). Dacie and Lewis practical haematology. Philadelphia: Churchill Livingstone/Elsevier. pp. [page needed]. ISBN 0-443-06660-4.
- ^ Myler P; Fasel N (editors). (2008). Leishmania: After The Genome. Caister Academic Press. p. [page needed]. ISBN 978-1-904455-28-8. http://www.horizonpress.com/leish.
- ^ a b Soto, J., Toledo, J. T. (2007). "Oral miltefosine to treat new world cutaneous leishmaniasis". Lancet Infect Dis 7 (1): 7. doi:10.1016/S1473-3099(06)70665-X. PMID 17182338. http://linkinghub.elsevier.com/retrieve/pii/S1473-3099(06)70665-X.
- ^ Arevalo J, Ramirez L, Adaui V, et al. (June 2007). "Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis". J. Infect. Dis. 195 (12): 1846–51. doi:10.1086/518041. PMID 17492601. http://www.journals.uchicago.edu/doi/abs/10.1086/518041?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ^ Sundar S, Chakravarty J, Rai VK, et al. (September 2007). "Amphotericin B treatment for Indian visceral leishmaniasis: response to 15 daily versus alternate-day infusions". Clin. Infect. Dis. 45 (5): 556–61. doi:10.1086/520665. PMID 17682988. http://www.journals.uchicago.edu/doi/abs/10.1086/520665?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ^ Mueller M, Ritmeijer K, Balasegaram M, Koummuki Y, Santana MR, Davidson R (January 2007). "Unresponsiveness to AmBisome in some Sudanese patients with kala-azar". Trans. R. Soc. Trop. Med. Hyg. 101 (1): 19–24. doi:10.1016/j.trstmh.2006.02.005. PMID 16730363. http://linkinghub.elsevier.com/retrieve/pii/S0035-9203(06)00101-5.
- ^ van Thiel P.P.A.M. et al. (2010). "Miltefosine treatment of Leishmania major infection: an observational study involving Dutch military personnel returning from northern Afghanistan". Clinical Infectious Diseases 50 (1): 80–3. doi:10.1086/648726. PMID 19951107. http://www.journals.uchicago.edu/doi/abs/10.1086/648726.
- ^ Jha TK, Sundar S, Thakur CP et al. (1999). "Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis". New Engl J Med 341 (24): 1795–800. doi:10.1056/NEJM199912093412403. PMID 10588964.
- ^ More et al.; Bhatt, H; Kukreja, V; Ainapure, SS (2003). "Miltefosine: great expectations against visceral leishmaniasis". Journal of Postgraduate Medicine 49 (1): 101–3. doi:10.4103/0022-3859.911. PMID 12865588. http://www.jpgmonline.com/article.asp?issn=0022-3859;year=2003;volume=49;issue=1;spage=101;epage=3;aulast=More.
- ^ A Small Charity Takes the Reins in Fighting a Neglected Disease, New York Times, July 31, 2006.
- ^ "Drug Program - Clinical Trial of Paramomycin". Institute for OneWorld Health. http://www.oneworldhealth.org/drug_program. Retrieved 10 February 2011.
- ^ Sundar S, Agrawal N, Arora R, et al. (2009). "Short‐course paromomycin treatment of visceral leishmaniasis in India: 14‐day vs 21‐day treatment". Clin Infect Dis 49 (6): 850–851. doi:10.1086/605432. PMID 19673613. http://www.journals.uchicago.edu/doi/abs/10.1086/605438.
- ^ Badaro R, Lobo I, Munos A, et al. (October 2006). "Immunotherapy for drug-refractory mucosal leishmaniasis". J Infect Dis. 194 (8): 1151–9. doi:10.1086/507708. PMID 16991091. http://www.journals.uchicago.edu/doi/abs/10.1086/507708?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ^ Leishmania major: In vitro and in vivo anti-leishmanial effect of cantharidin Ghaffarifar F. Experimental Parasitology 2010 126:2 (126-129)
- ^ Leishmaniasis: Magnitude of the problem. World Health Organization.
- ^ a b "Hope for tropical disease vaccine". BBC News. April 23, 2006. http://news.bbc.co.uk/1/hi/health/4930528.stm.
- ^ Visceral leishmaniasis. Institute for OneWorld Health.
- ^ "Dallas News: Rare, non-fatal skin disease found in N. Texans". http://www.dallasnews.com/sharedcontent/dws/dn/latestnews/stories/091507dnmetskin.d3fcc2e2.html. Retrieved 2009-10-05.
- ^ "Papalomoyo". http://www.almanaqueept.net/Publicaciones/1985/198525.pdf. Retrieved 2010-08-16.
- ^ leishmaniasis un brote serio[dead link]
- ^ Nota interior[dead link]
- ^ Welcome to www.serviciojesuitaarefugiados-vzla.org[dead link]
- ^ Al Jazeera English - CENTRAL/S. ASIA - Kabul: A city in intensive care
- ^ e-Ariana - Todays Afghan News
- ^ e-Ariana - Todays Afghan News
- ^ World Health Organization action in Afghanistan aims to control debilitating leishmaniasis.
- ^ Kennedy, Kelly (30 March 2010). "VCS Advocacy in the News: VA May Designate 9 Infectious Diseases as Related to Gulf War". Veterans for Common Sense. http://www.veteransforcommonsense.org/index.php/veterans-category-articles/1649-kelly-kennedy. Retrieved 10 February 2011.
- ^ Business: Company's mesh will help troops beat 'Baghdad boils'
- ^ http://www.pdhealth.mil/downloads/Leishmaniasis_exsu_16Mar042.pdf
- ^ Cox, Francis E G (1996). The Wellcome Trust illustrated history of tropical diseases. London: The Wellcome Trust. pp. 206–217. ISBN 1869835867, 9781869835866. OCLC 35161690. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=126866#id2621583.
- ^ "WHO: Leishmaniasis background information - a brief history of the disease". http://www.who.int/leishmaniasis/en/.
- ^ Cunningham, DD (1885). On the presence of peculiar parasitic organisms in the tissue of a specimen of Delhi boil. Scientific memoirs officers Medical Sanitary Departments Government India. Calcutta: Printed by the superintendent of government printing, India. pp. 21–31. OCLC 11826455.
- ^ Cox FE (2002). "History of Human Parasitology". Clin. Microbiol. Rev. 15 (4): 595–612. doi:10.1128/CMR.15.4.595-612.2002. PMC 126866. PMID 12364371. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=126866.
- ^ Hoare C.A. (1938). "Early discoveries regarding the parasite of oriental sore". Transactions of the Royal Society of Tropical Medicine and Hygiene 32 (1): 67–92. doi:10.1016/S0035-9203(38)90097-5.
- ^ Leishman, W. B. (1903). "On the possibility of the occurrence of trypanomiasis in India". The British Medical Journal.
- ^ Donovan, C. (1903). "Memoranda: On the possibility of the occurence of trypanomiasis in India". The British Medical Journal.
- ^ R. Ross (1903). "Further notes on Leishman's bodies". Ibid.: ii: 1401.
- ^ C. A. Bentley (24 December 1903). "Telegram to R. Ross". Ross Archives: 47/157.
- ^ "Leonard Goodwin - Telegraph". The Daily Telegraph. 14 January 2009. http://www.telegraph.co.uk/news/obituaries/4241645/Leonard-Goodwin.html. Retrieved 2009-01-18.
- ^ World Health Organization Initiative for Vaccine Research
- ^ Ivens AC, et al. (2005). "The Genome of the Kinetoplastid Parasite, Leishmania major". Science 309 (5733): 436–42. doi:10.1126/science.1112680. PMC 1470643. PMID 16020728. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1470643.
- ^ $5m for disease control in Ethiopia in Israel 21c Innovation News Service Retrieved 2009-12-30
- ^ Trudel N. et al. (2008). "Intracellular survival of Leishmania species that cause visceral leishmaniasis is significantly reduced by HIV-1 protease inhibitors". Journal of Infectious Diseases 198 (9): 1292–1299. doi:10.1086/592280. PMID 18816190.
- ^ Fogle catches a flesh-eating bug
External links
- Doctors Without Borders' leishmaniasis information page
- Drugs for Neglected Diseases Initiative
- International Leishmania Network
- Leish-L discussion list
- World Health Organization's leishmaniasis e-compendium
- A case-study by a young sufferer.
- Lainson, R and Range, E.L. (2005). "Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil, a review". Memórias do Instituto Oswaldo Cruz 100 (8): 811–27. doi:10.1590/S0074-02762005000800001. PMID 16444411.
- "Wanted: social entrepreneurs". Nature 434 (7036): 941. April 2005. doi:10.1038/434941a. PMID 15846306.
- "Leishmaniasis Symposium issue". Journal of Postgraduate Medicine 49 (1). 2003. http://www.jpgmonline.com/showbackIssue.asp?issn=0022-3859;year=2003;volume=49;issue=1.
- Leishmaniasis at the Open Directory Project
Infectious diseases – Parasitic disease: protozoan infection: Excavata (A06–A07, B55–B57, 007, 085–086) Discicristata TrypanosomatidaLeishmaniasisLeishmania major/L. mexicana/L. aethiopica/L. tropica (Cutaneous leishmaniasis) · L. braziliensis (Mucocutaneous leishmaniasis) · L. donovani/infantum (Visceral leishmaniasis)SchizopyrenidaTrichozoa TrichomonadidaCategories:- Protozoal diseases
- Zoonoses
- Tropical diseases
- Insect-borne diseases
- Parasitic infestations, stings, and bites of the skin
- Parasitic protists
Wikimedia Foundation. 2010.