- Lactone
-
In chemistry, a lactone is a cyclic ester[1] which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule. It is characterized by a closed ring consisting of two or more carbon atoms and a single oxygen atom, with a ketone group =O in one of the carbons adjacent to the other oxygen.
Contents
Nomenclature
Lactones are usually named according to the precursor acid molecule (aceto = 2 carbons, propio = 3, butyro = 4, valero = 5, capro = 6, etc.), with a -lactone suffix and a Greek letter prefix that specifies the number of carbons in the heterocyle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lactone = 4-membered, γ-lactone = 5-membered, etc.
The other suffix used to denote a lactone is -olide, used in substance class names like butenolide, macrolide, cardenolide or bufadienolide.
Etymology
The name lactone derives from the ring compound called lactide, which is formed from the dehydration of 2-hydroxypropanoic acid (lactic acid) CH3-CH(OH)-COOH. Lactic acid, in turn, derives its name from its original isolation from soured milk (Latin: lac, lactis). An internal dehydration within the same molecule of lactic acid would have produced alpha-propiolactone, a lactone with a 3-membered ring.
Natural sources
Lactones (specifically 3-methyl-4-octanolide) are found in oak trees as well as many other plants, and impart flavour to whisky.[citation needed]
Synthesis
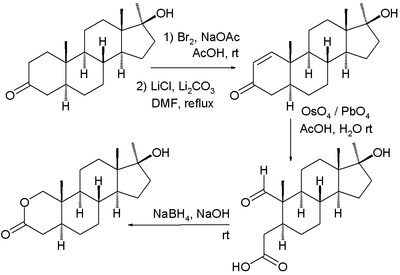
Many methods in ester synthesis can also be applied to that of lactones. In one industrial synthesis of oxandrolone the key lactone-forming step is an organic reduction - esterification:[2][3]
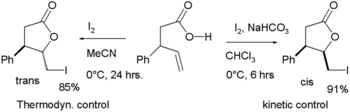
In halolactonization, an alkene is attacked by a halogen via electrophilic addition with the cationic intermediate captured intramolecularly by an adjacent carboxylic acid (See also iodolactamization), for example in this iodolactonization:[4]
A specific method is called Yamaguchi esterification.
A recent study has isolated β-lactones from bromination of 2,3-dimethylmaleate and/or 2,3-dimethylfumarate disodium salts, under ambient and aqueous conditions. The carboxylate groups of the maleate and fumarate moieties exhibit neighbouring group effects and alpha-lactones are proposed in the detailed mechanism.[5]
Reactions
The most stable structure for lactones are the 5-membered γ-lactones and 6-membered δ-lactones because, as in all organic cycles, 5 and 6 membered rings minimize the strain of bond angles. γ-lactones are so stable that, in the presence of dilute acids at room temperature, 4-hydroxy acids (R-CH(OH)-(CH2)2-COOH) immediately undergo spontaneous esterification and cyclisation to the lactone. β-lactones do exist, but can only be made by special methods. α-lactones can be detected as transient species in mass spectrometry experiments.[6]
The reactions of lactones are similar to those of esters, as exemplified by gamma-lactone in the following sections:
Hydrolysis
Heating a lactone with a base (sodium hydroxide) will hydrolyse the lactone to its parent compound, the straight chained bifunctional compound. Like straight-chained esters, the hydrolysis-condensation reaction of lactones is a reversible reaction, with an equilibrium. However, the equilibrium constant of the hydrolysis reaction of the lactone is lower than that of the straight-chained ester i.e. the products (hydroxyacids) are less favored in the case of the lactones. This is because although the enthalpies of the hydrolysis of esters and lactones are about the same, the entropy of the hydrolysis of lactones is less than the entropy of straight-chained esters. Straight-chained esters give two products upon hydrolysis, making the entropy change more favorable than in the case of lactones which give only a single product.
Reduction
Lactones can be reduced to diols using lithium aluminium hydride in dry ether. The reduction reaction will first break the ester bond of the lactone, and then reduce the carboxylic acid group (-COOH) to the alcohol group (-OH). For instance, gamma-lactones will be reduced to butan-1,4-diol, (CH2(OH)-(CH2)2-CH2(OH).
Aminolysis
Lactones also react with ethanolic ammonia, which will first break the ester bond and then react with the acidic -COOH group, because of the basic properties of ammonia, to form a difunctional group, i.e. alcohol and amide. Gamma-lactones will react to yield CH2(OH)-(CH2)2-CO-NH2.
Michael reaction
Sesquiterpene lactones, found in many plants, can react with other molecules via a Michael reaction.
Industrial Uses
Biofilm prevention
Brominated furanones have been shown to be somewhat effective at preventing the formation of biofilm. One species has specifically been shown to increase Salmonella enterica serovar Typhimurium's susceptibility to antimicrobial treatments.[7]
Examples
-
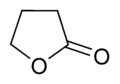
γ-butyrolactone (GBL)
dilactones
- Ellagic acid (Hexahydroxydiphenic acid dilactone)
- Flavogallonic acid dilactone can be found in Rhynchosia volubilis seeds and in Shorea laeviforia
- Lactide
- Tergallic acid dilactone can be found in Rhynchosia volubilis seeds
- Valoneic acid dilactone can be isolated from the heartwood of Shorea laeviforia
See also
References & notes
- ^ March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure Michael B. Smith, Jerry March Wiley-Interscience, 5th edition, 2001, ISBN 0-471-58589-0
- ^ Development of a Commercial Process to Produce Oxandrolone John E. Cabaj, David Kairys, and Thomas R. Benson Org. Process Res. Dev.; 2007; 11(3) pp 378 - 388; (Article) doi:10.1021/op060231b
- ^ The complete reaction sequence is bromination to a haloketone (not displayed), elimination reaction with lithium chloride to an enone, organic oxidation by osmiumtetroxide and lead tetraacetate with ring-opening and finally reduction of the aldehyde to the alcohol with sodium borohydride and intramolecular lactone formation
- ^ Organic Syntheses, Coll. Vol. 7, p.164 (1990); Vol. 64, p.175 (1986) Article link.
- ^ Chem. Commun., 2001, 485-486, DOI: 10.1039/b100335f Article link
- ^ Detlef Schröder, Norman Goldberg, Waltraud Zummack, Helmut Schwarz, John C. Poutsma and Robert R. Squires (1997), Generation of α-acetolactone and the acetoxyl diradical •CH2COO• in the gas phase. International Journal of Mass Spectrometry and Ion Processes, Volumes 165-166, November issue, Pages 71-82. doi:10.1016/S0168-1176(97)00150-X
- ^ Janssens JC, Steenackers H, Robijns S, Gellens E, et. al. (2008), Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl Environ Microbiol. 2008 Nov. 74(21):6639-48. PMID:18791004
Categories:- Lactones
- Functional groups
Wikimedia Foundation. 2010.