- Trypanosoma cruzi
Taxobox
color = khaki
name = "Trypanosoma cruzi"
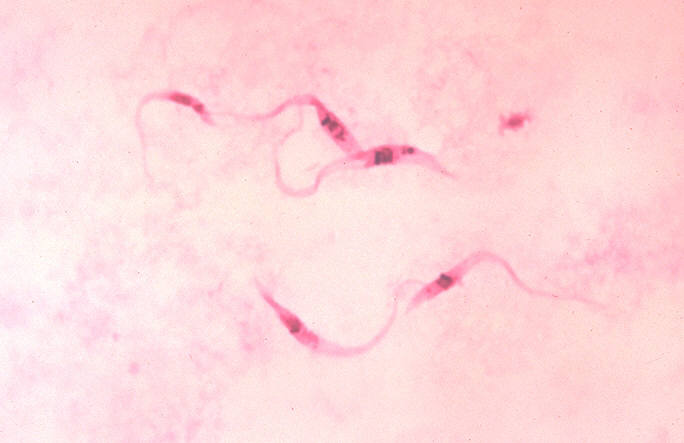
image_caption = "Trypanosoma cruzi", crithidia.
domain =Eukarya
regnum =Protist a
phylum =Euglenozoa
classis =Kinetoplastida
ordo =Trypanosomatida
genus = "Trypanosoma "
species = "T. cruzi""
Trypanosoma cruzi" is a species of parasiticeuglenoid trypanosome s. The species causes thetrypanosomiasis diseases in humans and animals in America. Transmission occurs when the reduviid bug deposits feces on the skin surface and subsequently bites; the human host then scratches the bite area and facilitiates penetration of the infected feces.Human American Trypanosomiasis, or
Chagas disease , is a potentially fatal disease of humans. It has two forms, a trypomastigote found in human blood and an amastigote found in tissues. The acute form usually goes unnoticed and may present as a localized swelling at the site of entry of the parasites in the skin. The chronic form may develop 10 to 20 years after infection. This form affects internal organs (e.g. theheart ,esophagus , colon and theperipheral nervous system ). Affected people may die from heart failure.Acute cases are treated with
nifurtimox andbenznidazole , but there is currently no effective therapy for chronic cases.Life cycle
Trypanosoma cruzi's life cycle starts in an animal reservoir. These reservoirs are usually mammals, wild or domestic, and include humans. A triatomine bug serves as the vector. While taking a blood meal, the triatomine sucks up the T. cruzi. In the triatomine bug, they go into the epimastigote stage. This makes it possible to reproduce. After reproducing through mitosis, the epimastigotes move onto the rectal cell wall. There, they become infectious. Infectious T. cruzi are called trypomastigotes. Then, while the triatomine bug is taking a blood meal from a human, it defecates. The trypomastigotes are in the feces.
The trypomastigotes enter the human host through the bite wound or by crossing mucous membranes. When they enter a human cell, they become amastigotes. This is another reproductive stage. After reproducing through mitosis until a large amount of amastigotes are in a cell, pseudocysts are formed in infected cells. The amastigotes then turn back into trypomastigotes, and the cell bursts. The typomastigotes swim along to either infect other cells or get sucked up by other triatomine bugs.
Pathophysiology
Myocardial biochemical response
Subcellular findings in murine studies with induced T. cruzi infection revealved that the chronic state is associated with the persistent elevation of phosporylated (activated) extracellular-signal-regulated kinase (
ERK ),AP-1 , andNF-κB . Also, the mitotic regulator for the G1 progression,cyclin D1 , was found activated. It is indicated that although there was no increase in any isoform of ERK, there was an increased concentration of phosphorylated ERK in T. cruzi infected mice. It was found that within 7 days the concentration of AP-1 was significantly higher in T. cruzi infected mice when compared to the control. elevated levels of NF-κB have also been found in myocardial tissue the highest concentrations being found in the vasculature. It was indicated throughWestern blot that cyclin D1 was upregulated from day 1 to day 60 post infection. It was also indicated through immunohistochemistry that the areas that produced the most cyclin D1 were the vasculature and interstitial regions of the heart. [cite journal | author = Huang H, Petkova SB, Cohen AW et al | title = Activation of transcription factors AP-1 and NF-kappa B in murine Chagasic mycarditis | journal = Infect. Immun. | volume = 71 | issue = 5 | pages = 2859–67 | year = 2003 | pmid = 12704159 | doi = 10.1128/IAI.71.5.2859-2867.2003]Conduction abnormalities
Also associated with [] are conduction abnormalities. At the base of these conduction abnormalities is a depopulation of parasympathetic neuronal endings on the heart. Without proper parasympathetic innervations, one could expect to find not only
chronotropic but alsoionotropic abnormalities. It is true that all inflammatory and non-inflammatory heart disease may display forms of parasympathetic denervation, this denervation presents in a descriptive fashion in Chagas’ disease. It has also been indicated that the loss of parasympathetic innervations can lead to sudden death. This sudden death is due to a severe cardiac failure that occurs during the acute stage of infection. [ Baroldi G et al. Sudden and unexpected death in clinically “silent” Chagas’ cardiomyopathy. International Journal of Cardiology. 1996 54:149-156. ]Another conduction abnormality presented with chronic Chagas’ disease is a change in ventricular repolarization. Ventricular repolarization is represented on an electrocardiogram as the T-wave. This change in repolarization inhibits the heart from relaxing and entering
diastole properly. Changes in the ventricular repolarization in Chagas’ disease are likely due to myocardialischemia . This ischemia can also lead tofibrillation . This sign is usually observed in chronic Chagas’ disease and is considered a minor electromyocardiopathy. [ Valente N. et al. Serial electrophysiological studies of the heart’s exicto conductor system in patients with chronic chagasic cardiopathy. Arq Bras Cardiol. 86(1): 19-25. 2006 ]Another class of electrocardiomyopathies associated with Chagas’ disease are the bundle branch blocks. These include incomplete left bundle branch block, complete left
bundle branch block and complete right bundle branch block. These defects occur because of a lack of conduction in the bundle branches which connect the AV node to thepurkinje fibers , which mediates a concerted contraction of the ventricles. A bundle branch block is usually associated with a change in theElectrocardiogram vector. The ECG vector usually runs straight to the apex of the heart, in a bundle branch block the vector will run to the opposite from the block. Chagasic bundle branch block is presented in chronic Chagas’ disease and is considered a moderate electrocardiomyopathy.Severe conduction abnormalities associated with Chagas’ disease occurs when a bundle branch block spreads past the
bundle of His and creates anAtrial-Ventricular block . At this point the patient will present with and impaired conduction velocity, mild bradycardia, or elicitWenckebach phenomenon depending to the degree of AV block. Also presented with severe Chagas’ cardiomyopathy but lying outside the scope of this subsection would bedyspnea andsyncope .Epicardial lesions
Also associated with Chagas’ disease are epicardial lesions. These lesions include Milk spots, Chagasic rosary, and villous plaque. It has been proposed that these three categories of lesions although occurring in different areas of the heart and having different histological appearances are all epicardial reactions to chronic inflammatory responses. These lesions could be the direct cause of conduction problems, thrombosis problems, or even the root of the ventricular remodeling.
Upon examination milk spots are characterized as white areas on ventricular epicardium, with precise borders, and often appear on the right ventricle. The microscopic anatomy of the milk spot it was observed that they are composed of parallel arrangements of densely compacted
collagen fibers with no vasculature. The location of these fibers may indicate that they interfere with the conduction of current through the heart. It has been proposed that milk spots are actually scars, due to the lack of inflammatory cells and vascular proliferation. Let it be noted that milk spots are not purely indicative of heart conditions related to Chagas’ disease, but they are also associated with many chronic heart diseases.Chagasic rosary refers to small round granules deposited along the coronary vessels. These small round granules are arranged like a string of beads, hence the term
rosary . The chagasic rosary structures are composed of the same collagen fibers that milk spots are formed from, the difference between the two are size and location. The location of chagasic rosary may play a role in thethromboembolism associated with Chagas’ disease. Chagasic rosary is unique to Chagas’ disease, however it should not be considered a pathognomonic lesion of the chagasic etiology.Villous plaque is characterized by exophytic
epicardial thickening, meaning that the growth is occurring at the border of the epicardium and not the center of mass. Unlike milk spots and chagasic rosary, villous plaque has inflammatory cells and vasculature present. Since villous plaque still contains inflammatory cells it is reasonable to suspect that these lesions were more recently formed compared to milk spots or chagasic rosary. [ Benvenuti L.A. & Gutierrez P.S. Epicardial Lesions in Chagas’ Heart Disease Reflect an Inflammatory Process. Arq Bras Cardiology. 2007 88(4): 438-440. ]Cardiac manifestations
Researchers of Chagas’ disease have demonstrated several processes that occur with all cardiomyopathies. The first event is an inflammatory response. Following the inflammation, cellular damage will occur. Finally in the body’s attempt to recover from the cellular damage, fibrosis will set into the cardiac tissue. [ Leiby et al. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002 42 ]
Chagas’ disease can affect myocardial function by causing heart failure syndrome. Depending on which side of the heart is affected by chagasic cardiomypopathies there will be different clinical manifestations throughout the body. Associated with right side damage will be edema, ascites, hepatomegaly, and pathologic jugular turgor. The previously mentioned symptoms are due to inadequate removal of venous blood. Associated with left side damage will be pulmonary congestion and low cardiac output. Also observed in heart failure syndrome is apical aneurysm, sometimes with a diameter between 2-5 cm, weakening the endocardial-pericardial junction.
Arrhythmic syndrome is also a cardiomyopathy clinically associated with Chagas’ disease. The alterations of the contactile rhythms spur from atrioventricular and intraventricular conduction defects, dysfunction of the sinus node, primary and secondary ventricular repolarization disturbance, fibrosis and inflammation, autonomic dysfunction, and endothelial dysfunction.
There is some evidence that chronic Chagas disease also can cause autonomic disfunction such as impaired regulation of heart rate in response to various physiological stresses such as orthostatic testing or the Valsalva maneuver.
Another cardiomyopathy found in nearly all cases of chronic Chagas’ disease is Thromoembolic syndrome. Thromboembolism describes thrombosis, the formation of a clot, and its main complication –embolism, the carrying of a clot to a distal section of the vessel and causing blockage. This occurrence contributes to the death of a patient by four means: arrhythmias, stasis secondary to cardiac dilation, mural endocarditis, and cardiac fibrosis. These thrombi also affect other organs such as the brain, spleen and kidney. [ Marin-Neto J.A. et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007 115(9): 1109-23 ]
References
External links
* [http://www.vcu.edu/csbc/vpp/parasites.html "VCU Virtual Parasite Project"] , T-cruzi parasite simulation
* [http://tcruzidb.org "TcruziDB"] , Integrated T-cruzi genome resource
Wikimedia Foundation. 2010.
