- Diatom
-
For a molecule of two atoms, see Diatomic molecule.
Diatoms 
Marine diatoms Scientific classification Domain: Eukaryota Kingdom: Chromalveolata Phylum: Heterokontophyta Class: Bacillariophyceae
Haeckel 1878Orders Diatoms[1] are a major group of algae, and are one of the most common types of phytoplankton. Most diatoms are unicellular, although they can exist as colonies in the shape of filaments or ribbons (e.g. Fragillaria), fans (e.g. Meridion), zigzags (e.g. Tabellaria), or stellate colonies (e.g. Asterionella). Diatoms are producers within the food chain. A characteristic feature of diatom cells is that they are encased within a unique cell wall made of silica (hydrated silicon dioxide) called a frustule. These frustules show a wide diversity in form, but usually consist of two asymmetrical sides with a split between them, hence the group name. Fossil evidence suggests that they originated during, or before, the early Jurassic Period. Diatom communities are a popular tool for monitoring environmental conditions, past and present, and are commonly used in studies of water quality.
Contents
General biology
There are more than 200 genera of living diatoms, and it is estimated that there are approximately 100,000 extant species.[2][3][4][5] Diatoms are a widespread group and can be found in the oceans, in freshwater, in soils and on damp surfaces. Most live pelagically in open water, although some live as surface films at the water-sediment interface (benthic), or even under damp atmospheric conditions. They are especially important in oceans, where they are estimated to contribute up to 45% of the total oceanic primary production.[citation needed] Spatial distribution of marine phytoplankton species is restricted both horizontally and vertically.[6][7] Usually microscopic, some species of diatoms can reach up to 2 millimetres in length.
Diatoms belong to a large group called the heterokonts, including both autotrophs (e.g. golden algae, kelp) and heterotrophs (e.g. water moulds). Their yellowish-brown chloroplasts are typical of heterokonts, with four membranes and containing pigments such as the carotenoid fucoxanthin. Individuals usually lack flagella, but they are present in gametes and have the usual heterokont structure, except they lack the hairs (mastigonemes) characteristic in other groups. Most diatoms are non-motile, although some move via flagellation. As their relatively dense cell walls cause them to readily sink, planktonic forms in open water usually rely on turbulent mixing of the upper layers by the wind to keep them suspended in sunlit surface waters. Some species actively regulate their buoyancy with intracellular lipids to counter sinking. A feature of diatoms is the urea cycle, which links them evolutionarily to animals.[8]
Diatom cells are contained within a unique silicate (silicic acid) cell wall comprising two separate valves (or shells). The biogenic silica that the cell wall is composed of is synthesised intracellularly by the polymerisation of silicic acid monomers. This material is then extruded to the cell exterior and added to the wall. Diatom cell walls are also called frustules or tests, and their two valves typically overlap one over the other like the two halves of a petri dish. In most species, when a diatom divides to produce two daughter cells, each cell keeps one of the two halves and grows a smaller half within it. As a result, after each division cycle the average size of diatom cells in the population gets smaller. Once such cells reach a certain minimum size, rather than simply divide vegetatively, they reverse this decline by forming an auxospore. This expands in size to give rise to a much larger cell, which then returns to size-diminishing divisions. Auxospore production is almost always linked to meiosis and sexual reproduction.
Decomposition and decay of diatoms leads to organic and inorganic (in the form of silicates) sediment, the inorganic component of which can lead to a method of analyzing past marine environments by corings of ocean floors or bay muds, since the inorganic matter is embedded in deposition of clays and silts and forms a permanent geological record of such marine strata.
The study of diatoms is a branch of phycology, and phycologists specializing in diatoms are called diatomists.
Classification
 Selections from Ernst Haeckel's 1904 Kunstformen der Natur (Art Forms of Nature), showing pennate (left) and centric (right) frustules.
Selections from Ernst Haeckel's 1904 Kunstformen der Natur (Art Forms of Nature), showing pennate (left) and centric (right) frustules.
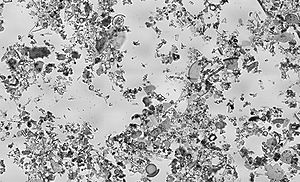 Diatomaceous earth as viewed under bright field illumination on a light microscope. Diatomaceous earth is a soft, siliceous, sedimentary rock made up of the cell walls of diatoms and readily crumbles to a fine powder. This sample consists of a mixture of centric (radially symmetric) and pennate (bilaterally symmetric) diatoms. This image of diatomaceous earth particles in water is at a scale of 6.236 pixels/μm, the entire image covers a region of approximately 1.13 by 0.69 mm.
Diatomaceous earth as viewed under bright field illumination on a light microscope. Diatomaceous earth is a soft, siliceous, sedimentary rock made up of the cell walls of diatoms and readily crumbles to a fine powder. This sample consists of a mixture of centric (radially symmetric) and pennate (bilaterally symmetric) diatoms. This image of diatomaceous earth particles in water is at a scale of 6.236 pixels/μm, the entire image covers a region of approximately 1.13 by 0.69 mm.
The classification of heterokonts is still unsettled, and they may be treated as a division (or phylum), kingdom, or something in-between. Accordingly, groups like the diatoms may be ranked anywhere from class (usually called Diatomophyceae) to division (usually called Bacillariophyta), with corresponding changes in the ranks of their subgroups.
Diatoms are traditionally divided into two orders:
- centric diatoms (Centrales), which are radially symmetric
- pennate diatoms (Pennales), which are bilaterally symmetric. The former are paraphyletic to the latter.
A more recent classification[3] divides the diatoms into three classes:
- centric diatoms (Coscinodiscophyceae)
- pennate diatoms
- without a raphe (Fragilariophyceae)
- with a raphe (Bacillariophyceae)
It is probable there will be further revisions as understanding of their relationships increases.[9]
Diatoms generally range in size from ca. 2-200μm,[2] and are composed of a cell wall composed primarily of silica.[7] This siliceous wall can be highly patterned with a variety of pores, ribs, minute spines, marginal ridges and elevations; all of which can be utilised to delineate genera and species. The cell itself consists of two halves, each containing an essentially flat plate, or valve and marginal connecting, or girdle band. One half, the hypotheca, is slightly smaller than the other half, the epitheca. Diatom morphology varies. Although the shape of the cell is typically circular, some cells may be triangular, square, or elliptical.
Cells are solitary or united into colonies of various kinds, which may be linked by siliceous structures; mucilage pads, or stalks; mucilage tubes; amorphous masses of mucilage and threads of polysaccharide (chitin), which are secreted through strutted processes. Major pigments of diatoms are chlorophylls a and c, beta-carotene, fucoxanthin, diatoxanthin and diadinoxanthin.[2] Diatoms are primarily photosynthetic. A few, however, are obligate heterotrophs, while others can live heterotrophically in the absence of light, provided an appropriate organic carbon source is available. Storage products are chrysolaminarin and lipids.[7]
Round & Crawford (1990)[3] and Hoek et al. (1995)[10] provide more comprehensive coverage of diatom taxonomy.
Ecology
 A budget of the ocean's silicon cycle[11]
A budget of the ocean's silicon cycle[11]
Planktonic diatoms in freshwater and marine environments typically exhibit a "boom and bust" (or "bloom and bust") lifestyle. When conditions in the upper mixed layer (nutrients and light) are favourable (e.g. at the start of spring) their competitive edge[12] allows them to quickly dominate phytoplankton communities ("boom" or "bloom"). As such they are often classed as opportunistic r-strategists (i.e. those organisms whose ecology is defined by a high growth rate, r).
When conditions turn unfavourable, usually upon depletion of nutrients, diatom cells typically increase in sinking rate and exit the upper mixed layer ("bust"). This sinking is induced by either a loss of buoyancy control, the synthesis of mucilage that sticks diatoms cells together, or the production of heavy resting spores. Sinking out of the upper mixed layer removes diatoms from conditions unfavourable to growth, including grazer populations and higher temperatures (which would otherwise increase cell metabolism). Cells reaching deeper water or the shallow seafloor can then rest until conditions become more favourable again. In the open ocean, many sinking cells are lost to the deep, but refuge populations can persist near the thermocline.
Ultimately, diatom cells in these resting populations re-enter the upper mixed layer when vertical mixing entrains them. In most circumstances, this mixing also replenishes nutrients in the upper mixed layer, setting the scene for the next round of diatom blooms. In the open ocean (away from areas of continuous upwelling[13]), this cycle of bloom, bust, then return to pre-bloom conditions typically occurs over an annual cycle, with diatoms only being prevalent during the spring and early summer. In some locations, however, an autumn bloom may occur, caused by the breakdown of summer stratification and the entrainment of nutrients while light levels are still sufficient for growth. Since vertical mixing is increasing, and light levels are falling as winter approaches, these blooms are smaller and shorter-lived than their spring equivalents.
In the open ocean, the condition that typically causes diatom (spring) blooms to end is a lack of silicon. Unlike other nutrients, this is only a major requirement of diatoms so it is not regenerated in the plankton ecosystem as efficiently as, for instance, nitrogen or phosphorus nutrients. This can be seen in maps of surface nutrient concentrations - as nutrients decline along gradients, silicon is usually the first to be exhausted (followed normally by nitrogen then phosphorus).
Because of this bloom-and-bust cycle, diatoms are believed to play a disproportionately important role in the export of carbon from oceanic surface waters[13][14] (see also the biological pump). Significantly, they also play a key role in the regulation of the biogeochemical cycle of silicon in the modern ocean.[11][15]
 Egge & Aksnes (1992)[16] figure.
Egge & Aksnes (1992)[16] figure.
The use of silicon by diatoms is believed by many researchers to be the key to their ecological success. In a now classic study, Egge & Aksnes (1992)[16] found that diatom dominance of mesocosm communities was directly related to the availability of silicic acid — when concentrations were greater than 2 mmol m−3, they found that diatoms typically represented more than 70% of the phytoplankton community. Raven (1983)[17] noted that, relative to organic cell walls, silica frustules require less energy to synthesize (approximately 8% of a comparable organic wall), potentially a significant saving on the overall cell energy budget. Other researchers[18] have suggested that the biogenic silica in diatom cell walls acts as an effective pH buffering agent, facilitating the conversion of bicarbonate to dissolved CO2 (which is more readily assimilated). Notwithstanding the possible advantages conferred by silicon, diatoms typically have higher growth rates than other algae of a corresponding size.[12]
Diatoms occur in virtually every environment that contains water. This includes not only oceans, seas, lakes and streams, but also soil.
Life-cycle
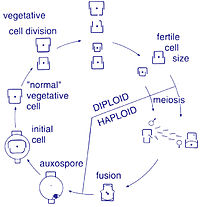 Sexual reproduction of a centric diatom (oogamy)
Sexual reproduction of a centric diatom (oogamy)
Diatoms are non-motile; however, sperm found in some species can be flagellated, though motility is usually limited to a gliding motion.[7] Reproduction among these organisms is primarily asexual by binary fission, with each daughter cell receiving one of the parent cell's two frustules (or theca). This is used by each daughter cell as the larger frustule (or epitheca) into which a second, small frustule (or hypotheca) is constructed.
This form of division results in a size reduction of the daughter cell that received the smaller frustule from the parent and therefore the average cell size of a diatom population decreases, until the cells are about one-third their maximum size.[2] It has been observed, however, that certain taxa have the ability to divide without causing a reduction in cell size.[19] Nonetheless, in order to restore the cell size of a diatom population for those that do endure size reduction, sexual reproduction and auxospore formation must occur.[2] Vegetative cells of diatoms are diploid (2N) and so meiosis can take place, producing male and female gametes which then fuse to form the zygote. The zygote sheds its silica theca and grows into a large sphere covered by an organic membrane, the auxospore. A new diatom cell of maximum size, the initial cell, forms within the auxospore thus beginning a new generation. Resting spores may also be formed as a response to unfavourable environmental conditions with germination occurring when conditions improve.[7]
In centric diatoms, the small male gametes have one flagellum while the female gametes are large and non-motile (oogamous). Conversely, in pinnate diatoms both gametes lack flagella (isoogamous).[2] Certain araphid species have been documented as anisogamous and are, therefore, considered to represent a transitional stage between centric and pinnate diatoms.[19]
Evolutionary history
Heterokont chloroplasts appear to be derived from those of red algae, rather than directly from prokaryotes as occurred in plants. This suggests they had a more recent origin than many other algae. However, fossil evidence is scant, and it is really only with the evolution of the diatoms themselves that the heterokonts make a serious impression on the fossil record.
The earliest known fossil diatoms date from the early Jurassic (~185 Ma),[20] although molecular clock[20] and sedimentary[21] evidence suggests an earlier origin. It has been suggested that their origin may be related to the end-Permian mass extinction (~250 Ma), after which many marine niches were opened.[22] The gap between this event and the time that fossil diatoms first appear may indicate a period when diatoms were unsilicified and their evolution was cryptic.[23] Since the advent of silicification, diatoms have made a significant impression on the fossil record, with major deposits of fossil diatoms found as far back as the early Cretaceous, and some rocks (diatomaceous earth, diatomite, kieselguhr) being composed almost entirely of them.
Although the diatoms may have existed since the Triassic, the timing of their ascendancy and "take-over" of the silicon cycle is more recent. Prior to the Phanerozoic (before 544 Ma), it is believed that microbial or inorganic processes weakly regulated the ocean's silicon cycle.[24][25][26] Subsequently, the cycle appears dominated (and more strongly regulated) by the radiolarians and siliceous sponges, the former as zooplankton, the latter as sedentary filter feeders primarily on the continental shelves.[27] Within the last 100 My, it is thought that the silicon cycle has come under even tighter control, and that this derives from the ecological ascendancy of the diatoms.
However, the precise timing of the "take-over" is unclear, and different authors have conflicting interpretations of the fossil record. Some evidence, such as the displacement of siliceous sponges from the shelves,[28] suggests that this takeover began in the Cretaceous (146 Ma to 65 Ma), while evidence from radiolarians suggests "take-over" did not begin until the Cenozoic (65 Ma to present).[29] The expansion of grassland biomes and the evolutionary radiation of grasses during the Miocene is believed to have increased the flux of soluble silicon to the oceans, and it has been argued that this has promoted the diatoms during the Cenozoic era.[30][31] However, work on the variation of diatom diversity during the Cenozoic suggests instead that diatom success is decoupled from the evolution of grasses, and that diatoms were most diverse prior to the diversification of grasses.[32] Nevertheless, regardless of the details of the "take-over" timing, it is clear that this most recent revolution has installed much tighter biological control over the biogeochemical cycle of silicon.
Fossil record
The fossil record of diatoms has largely been established through the recovery of their siliceous frustules in marine and non-marine sediments. Although diatoms have both a marine and non-marine stratigraphic record, diatom biostratigraphy, which is based on time-constrained evolutionary originations and extinctions of unique taxa, is only well developed and widely applicable in marine systems. The duration of diatom species ranges have been documented through the study of ocean cores and rock sequences exposed on land.[33] Where diatom biozones are well established and calibrated to the geomagnetic polarity time scale (e.g., Southern Ocean, North Pacific, eastern equatorial Pacific), diatom-based age estimates may be resolved to within <100,000 years, although typical age resolution for Cenozoic diatom assemblages is several hundred thousand years.
The Cretaceous record of diatoms is limited, but recent studies reveal a progressive diversification of diatom types. The Cretaceous-Tertiary extinction event, which in the oceans dramatically affected organisms with calcareous skeletons, appears to have had relatively little impact on diatom evolution.[34]
Although no mass extinctions of marine diatoms have been observed during the Cenozoic, times of relatively rapid evolutionary turnover in marine diatom assemblages occurred near the Paleocene–Eocene boundary[35] and at the Eocene–Oligocene boundary.[36] Further turnover of assemblages took place at various times between the middle Miocene and late Pliocene,[37] in response to progressive cooling of polar regions and the development of more endemic diatom assemblages. A global trend toward more delicate diatom frustules has been noted from the Oligocene to the Quaternary.[33] This coincides with an increasingly more vigorous circulation of the ocean’s surface and deep waters brought about by increasing latitudinal thermal gradients at the onset of major ice sheet expansion on Antarctica and progressive cooling through the Neogene and Quaternary towards a bipolar glaciated world. This drove the diatoms into uptaking silica more competitively (i.e., to use less silica in formation of their frustules). Increased mixing of the oceans renews silica and other nutrients necessary for diatom growth in surface waters, especially in regions of coastal and oceanic upwelling.
Collection
Living diatoms are often found clinging in great numbers to filamentous algae, or forming gelatinous masses on various submerged plants. Cladophora is frequently covered with Cocconeis, an elliptically shaped diatom; Vaucheria is often covered with small forms. Diatoms are frequently present as a brown, slippery coating on submerged stones and sticks, and may be seen to "stream" with river current.
The surface mud of a pond, ditch, or lagoon will almost always yield some diatoms. They can be made to emerge by filling a jar with water and mud, wrapping it in black paper and letting direct sunlight fall on the surface of the water. Within a day, the diatoms will come to the top in a scum and can be isolated.
Since diatoms form an important part of the food of molluscs, tunicates, and fishes, the alimentary tracts of these animals often yield forms that are not easily secured in other ways. Marine diatoms can be collected by direct water sampling, though benthic forms can be secured by scraping barnacles, oyster shells, and other shells.
This section uses text from Methods in Plant Histology.[38]
EST sequencing
The first insights into the properties of the P. tricornutum gene repertoire was described using 1,000 ESTs.[39] Subsequently, the number of ESTs was extended to 12,000 and the Diatom EST Database was constructed for functional analyses.[40] These sequences have been used to make a comparative analysis between P. tricornutum and the putative complete proteomes from the green alga Chlamydomonas reinhardtii, the red alga Cyanidioschyzon merolae, and T. pseudonana.[41] The diatom EST database now consists in over 200,000 ESTs from P. tricornutum (16 libraries) and T. pseudonana (7 libraries) cells grown in a range of different conditions, many of which corresponding to different abiotic stresses (available at http://www.biologie.ens.fr/diatomics/EST3/).[42]
Genome sequencing
The entire genomes of the centric diatom, Thalassiosira pseudonana (32.4 Mb),[43] and the pennate diatom, Phaeodactylum tricornutum (27.4 Mb),[44] have been sequenced. Comparisons of the two fully sequenced diatom genomes finds that the P. tricornutum genome includes fewer genes (10,402 opposed to 11,776) than T. pseudonana and no major synteny (gene order) could be detected between the two genomes. T. pseudonana genes show an average of ~1.52 introns per gene as opposed to 0.79 in P. tricornutum, suggesting recent widespread intron gain in the centric diatom.[44][45] Despite relatively recent evolutionary divergence (90 million years), the extent of molecular divergence between centrics and pennates indicates rapid evolutionary rates within the Bacillariophyceae compared to other eukaryotic groups.[44] Comparative genomics also established that a specific class of transposable elements, the Diatom Copia-like retrotransposons (or CoDis), has been significantly amplified in the P. tricornutum genome with respect to T. pseudonana, constituting 5.8 and 1% of the respective genomes.[46]
Importantly, diatom genomics brought much information about the extent and dynamics of the endosymbiotic gene transfer (EGT) process. Comparison of the T. pseudonana proteins with homologs in other organisms suggested that hundreds have their closest homologs in the Plantae lineage. EGT towards diatom genomes can be illustrated by the fact that the T. pseudonana genome encodes six proteins which are most closely related to genes encoded by the Guillardia theta (cryptomonad) nucleomorph genome. Four of these genes are also found in red algal plastid genomes, thus demonstrating successive EGT from red algal plastid to red algal nucleus (nucleomorph) to heterokont host nucleus.[43] More recent phylogenomic analyses of diatom proteomes provided evidence for a prasinophyte-like endosymbiont in the common ancestor of chromalveolates as supported by the fact the 70% of diatom genes of Plantae origin are of green lineage provenance and that such genes are also found in the genome of other stramenopiles. Therefore, it was proposed that chromalveolates are the product of serial secondary endosymbiosis first with a green algae, followed by a second one with a red algae that conserved the genomic footprints of the previous but displaced the green plastid.[47] However, phylogenomic analyses of diatom proteomes and chromalveolate evolutionary history will likely take advantage of complementary genomic data from under-sequenced lineages such as red algae.
In addition to EGT, horizontal gene transfer (HGT) can occur independently of an endosymbiotic event. The publication of the P. tricornutum genome reported that at least 587 P. tricornutum genes appear to be most closely related to bacterial genes, accounting for more than 5% of the P. tricornutum proteome. About half of these are also found in the T. pseudonana genome, attesting their ancient incorporation in the diatom lineage.[44]
Nanotechnology research
The deposition of silica by diatoms may also prove to be of utility to nanotechnology.[48] Diatom cells repeatedly and reliably manufacture valves of various shapes and sizes, potentially allowing diatoms to manufacture micro- or nano-scale structures which may be of use in a range of devices, including: optical systems; semiconductor nanolithography; and even using diatom valves as vehicles for drug delivery. Using an appropriate artificial selection procedure, diatoms that produce valves of particular shapes and sizes could be evolved in the laboratory, and then used in chemostat cultures to mass produce nanoscale components.[49] It has also been proposed that diatoms could be used as a component of solar cells, by substituting photosensitive titanium dioxide for the silicon dioxide normally used in the creation of cell walls.[50]
See also
References
- ^ diá-tom-os "cut in half" (= dichó-tom-os) — diá "through" or "apart" and the root of tém-n-ō "I cut". Alternation between e and o in verb root is ablaut.
- ^ a b c d e f Hasle, G.R.; Syvertsen,E.E. (1997). Marine Diatoms. In: Tomas, C.R. (1997). Identifying Marine Diatoms and Dinoflagellates. Academic Press. pp. 5–385.
- ^ a b c Round, F. E. and Crawford, R. M. (1990). The Diatoms. Biology and Morphology of the Genera, Cambridge University Press, UK.
- ^ Canter-Lund, H. and Lund, J.W.G. (1995). Freshwater Algae, Biopress Limited. ISBN 0-948737-25-5.
- ^ {{Mann, D. G. (1989). The species concept in diatoms Evolution|volume=24|issue=1|pages=1–22}}
- ^ Lipps, J.H. (1970). "Plankton Evolution". Diatoms occur in all oceans from the poles to the tropics; polar and subpolar regions contain relatively few species compared with temperate biota. Although tropical regions exhibit the greatest number of species, more abundant populations are found in polar to temperate regions. 24 (1): 1–22. doi:10.2307/2406711. JSTOR 2406711.
- ^ a b c d e Horner, R.A. (2002). A Taxonomic Guide to Some Common Marine Phytoplankton. BiopressLtd. pp. 25–30. ISBN 0948737654.
- ^ http://www.sciencedaily.com/releases/2011/05/110511133553.htm
- ^ "Medlin, L.K. & Kaczmarska, I. (2004) Evolution of the diatoms: V. Morphological and cytological support for major clade and taxonomic revision. Phycologia 43: 245-270.". http://epic.awi.de/epic/Main?puid=22572&lang=en.
- ^ Hoek, C. van den, Mann, D. G. and Jahns, H. M. (1995). Algae: An introduction to phycology, Cambridge University Press, UK.
- ^ a b Treguer, P., Nelson, D. M., Van Bennekom, A. J., DeMaster, D. J., Leynaert, A. and Queguiner, B. (1995). The silica balance in the world ocean: A reestimate. Science 268, 375-379.
- ^ a b Furnas, M. J. (1990). In situ growth rates of marine phytoplankton: Approaches to measurement, community and species growth rates. J. Plankton Res. 12, 1117-1151.
- ^ a b Dugdale, R. C. and Wilkerson, F. P. (1998). Silicate regulation of new production in the equatorial Pacific upwelling. Nature 391, 270-273.
- ^ Smetacek, V. S. (1985). Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance. Mar Biol. 84, 239-251.
- ^ Yool, A. and Tyrrell, T. (2003). Role of diatoms in regulating the ocean's silicon cycle. Global Biogeochemical Cycles 17, 1103, doi:10.1029/2002GB002018.
- ^ a b Egge, J. K. and Aksnes, D. L. (1992). Silicate as regulating nutrient in phytoplankton competition. Mar Ecol. Prog. Ser. 83, 281-289.
- ^ Raven, J. A. (1983). The transport and function of silicon in plants. Biol. Rev. 58, 179-207.
- ^ Milligan, A. J. and Morel, F. M. M. (2002). A proton buffering role for silica in diatoms. Science 297, 1848-1850.
- ^ a b Drebes, G. (1977). "Sexuality". Botanical Monographs 13: 250–283.
- ^ a b Kooistra, W. H. C. F. and Medlin, L. K. (1996). Evolution of the diatoms (Bacillariophyta) : IV. A reconstruction of their age from small subunit rRNA coding regions and the fossil record. Mol. Phylogenet. Evol. 6, 391-407.
- ^ Schieber, J., Krinsley, D. and Riciputi, L. (2000). Diagenetic origin of quartz silt in mudstones and implications for silica cycling. Nature 406, 981-985.
- ^ Medlin, L. K., Kooistra, W. H. C. F., Gersonde, R., Sims, P. A. and Wellbrock, U. (1997). Is the origin of the diatoms related to the end-Permian mass extinction? Nova Hedwegia 65, 1-11.
- ^ Raven, J. A. and Waite, A. M. (2004). The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytologist 162, 45-61.
- ^ Siever, R. (1991). Silica in the oceans: biological-geological interplay. In: Schneider, S. H., Boston, P. H. (eds.), Scientists On Gaia, The MIT Press, Cambridge MA, USA, pp. 287-295.
- ^ Kidder, D. L. and Erwin, D. H. (2001). Secular distribution of biogenic silica through the Phanerozoic: Comparison of silica-replaced fossils and bedded cherts at the series level. J. Geol. 109, 509-522.
- ^ Grenne, T. and Slack, J. F. (2003). Paleozoic and Mesozoic silica-rich seawater: evidence from hematitic chert (jasper) deposits. Geology 31, 319-322.
- ^ Racki, G. and Cordey, F. (2000). Radiolarian palaeoecology and radiolarites: is the present the key to the past? Earth-Science Reviews 52, 83-120.
- ^ Maldonado, M., Carmona, M. C., Uriz, J. M. and Cruzado, A. (1999). Decline in Mesozoic reef-building sponges explained by silicate limitation. Nature 401, 785-788.
- ^ Harper, H. E. and Knoll, A. H. (1975). Silica, diatoms, and Cenozoic radiolarian evolution. Geology 3, 175-177.
- ^ Falkowski, P.G.; et al. (2004). "The evolution of modern eukaryotic phytoplankton". Science 305 (5682): 354–60. doi:10.1126/science.1095964. PMID 15256663.
- ^ Kidder, D.L.; Gierlowski-Kordesch, E.H. (2005). "Impact of grassland radiation on the nonmarine silica cycle and Miocene diatomite". PALAIOS 20 (2): 198–206. doi:10.2110/palo.2003.p03-108.
- ^ Rabosky, D.L.; Sorhannus, U. (2009). "Diversity dynamics of marine planktonic diatoms across the Cenozoic". Nature 457 (7226): 183–6. doi:10.1038/nature07435. PMID 19129846.
- ^ a b Scherer, R.P., Gladenkov, A.Yu., and Barron, J.A. (2007). Methods and applications of Cenozoic marine diatom biostratigraphy. “Paleontological Society Papers” 13, 61-83
- ^ Harwood, D.M., Nikolaev, V.A., and Winter, D.M. (2007). Cretaceous record of diatom evolution, radiation, and expansion. “Paleontological Society Papers” 13, 33-59
- ^ Strelnikova, N. I. (1990). Evolution of diatoms during the Cretaceous and Paleogene periods. In: Simola, H. (ed.), “Proceedings of the Tenth International Diatom Symposium”, Koeltz Scientific Books, Koenigstein, Germany, pp. 195-204
- ^ Baldauf, J.G. (1993). Middle Eocene through early Miocene diatom floral turnover. In: Prothero D., Berggren, W.H., (eds.), “Eocene-Oligocene climatic and biotic evolution”, Princeton University Press, Princeton, NJ, USA, pp 310-326
- ^ Barron, J.A. (2003). Appearance and extinction of planktonic diatoms during the past 18 m.y. in the Pacific and Southern oceans. “Diatom Research” 18, 203-224
- ^ Chamberlain, C. J. (1901) Methods in Plant Histology, University of Chicago Press, USA
- ^ Scala, S.; Carels, N., Falciatore, A., Chiusano, M.L. and Bowler, C. (July 2002). "Genome Properties of the Diatom Phaeodactylum tricornutum". Plant Physiol. 129 (3): 993–1002. doi:10.1104/pp.010713. PMC 166495. PMID 12114555. http://www.plantphysiol.org/cgi/content/full/129/3/993.
- ^ Maheswari, U., Montsant, A., Goll, J., Krishnasamy, S., Rajyashri, K.R., Patell, V.M. and Bowler, C. (2005). The Diatom EST Database. Nucleic Acids Research 33, 344–347.
- ^ Montsant, A.; Jabbari, K., Maheswari, U. and Bowler, C. (February 2005). "Comparative Genomics of the Pennate Diatom Phaeodactylum tricornutum". Plant Physiol. 137 (2): 500–13. doi:10.1104/pp.104.052829. PMC 1065351. PMID 15665249. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1065351.
- ^ Maheswari U, Mock T, Armbrust EV, Bowler C: Update of the Diatom EST Database: a new tool for digital transcriptomics. Nucleic Acids Res 2008.
- ^ a b Armbrust et al. (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306, 79-86.
- ^ a b c d Bowler, C.; et al. (2008). "The Phaeodactylum genome reveals the evolutionary history of diatom genomes". Nature 456 (7219): 239–244. doi:10.1038/nature07410. PMID 18923393.
- ^ Roy SW, Penny D. A very high fraction of unique intron positions in the intron-rich diatom Thalassiosira pseudonana indicates widespread intron gain. Mol Biol Evol. 2007 Jul;24(7):1447-57. Epub 2007 Mar 8. PubMed PMID: 17350938.
- ^ Maumus F, Allen AE, Mhiri C, Hu H, Jabbari K, Vardi A, Grandbastien MA, Bowler C. Potential impact of stress activated retrotransposons on genome evolution in a marine diatom. BMC Genomics. 2009 Dec 22;10:624.
- ^ Moustafa A, Beszteri B, Maier UG, Bowler C, Valentin K, Bhattacharya D: Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 2009, 324:1724-1726.
- ^ Bradbury, J. (2004). Nature's Nanotechnologists: Unveiling the Secrets of Diatoms. PLoS Biology 2, 1512-1515.
- ^ Drum, R.W.; Gordon, R. (2003). "Star Trek replicators and diatom nanotechnology". Trends Biotechnology 21 (8): 325–328. doi:10.1016/S0167-7799(03)00169-0. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCW-48XCHCX-3&_user=126770&_coverDate=08%2F31%2F2003&_rdoc=2&_fmt=high&_orig=browse&_srch=doc-info(%23toc%235181%232003%23999789991%23441282%23FLA%23display%23Volume)&_cdi=5181&_sort=d&_docanchor=&_ct=10&_acct=C000010399&_version=1&_urlVersion=0&_userid=126770&md5=5ba3a25106d2c6331a217a70291cbafd.
- ^ Johnson, R.C. (9 April 2009). "Diatoms could triple solar cell efficiency". EE Times. http://www.eetimes.com/showArticle.jhtml?articleID=216500176. Retrieved 13 April 2009.
External links
- Catalogue of Diatom Names, California Academy of Sciences
- Diatom Genome, Joint Genome Institute
- Diatom EST database, École Normale Supérieure
- Plankton*Net, taxonomic database including images of diatom species
- Life History and Ecology of Diatoms, University of California Museum of Paleontology
- Diatoms: 'Nature's Marbles', Eureka site, University of Bergen
- Diatom life history and ecology, Microfossil Image Recovery and Circulation for Learning and Education (MIRACLE), University College London
- Diatom page, Royal Botanic Garden Edinburgh
- Geometry and Pattern in Nature 3: The holes in radiolarian and diatom tests
- Art Deco Diatoms, Wim van Egmond
- Diatom QuickFacts, Monterey Bay Aquarium Research Institute
- Algae image database Academy of Natural Sciences in Philadelphia (ANSP)
- Diatom taxa Academy of Natural Sciences in Philadelphia (ANSP)
- Now, energy from single celled algae Deccan Herald
- Computer simulations of pattern formation in diatoms
SAR: Chromalveolata: Heterokont Ochrophyta/
(photosynthetic)PhaeistaHypogyristeaPinguiophyceae · Pelagophyceae · Actinochryso-/Dictyochophyceae: Florenciellales · Dictyochales · Rhizochromulinales · PedinellalesChrysistaEustigmataceae · Monodopsidaceae · PseudocharaciopsidaceaeAscoseirales · Cutleriales · Desmarestiales · Dictyotales · Discosporangiales · Ectocarpales · Fucales · Ishigeales · Laminariales · Nemodermatales · Onslowiales · Ralfsiales · Scytosiphonales · Scytothamnales · Sphacelariales · Sporochnales · Syringodermatales · TilopteridalesPhaeothamniophyceaePhaeothamniales · PleurochloridellalesChattonella, Fibrocapsa, Gonyostomum, Haramonas, Heterosigma, VacuolariaHeterogloeales · Ochromonadales · Rhizochloridales · SynuralesBotrydiales · Mischococcales · Tribonematales · VaucherialesKhakistaBacillariophytaBiddulphiophycidae · Chaetocerotophycidae · Corethrophycidae · Coscinodiscophycidae · Cymatosirophycidae · Lithodesmiophycidae · Rhizosoleniophycidae · ThalassiosirophycidaeBacillariophyceaeBacillariales · NaviculalesFragilariophyceaeFragilarialesFungus-like/
(nonphotosynthetic)PseudofungiBigyraOpalinata/SlopalinidaCategories:- Algae classes
- Biological oceanography
- Diatoms
- Planktology
Wikimedia Foundation. 2010.





