- Diazomethane
-
Diazomethane 
 Diazomethane
DiazomethaneIdentifiers CAS number 334-88-3 
PubChem 9550 ChemSpider 9176 
KEGG C19387 
Jmol-3D images Image 1 - [N-]=[N+]=C
Properties Molecular formula CH2N2 Molar mass 42.04 g/mol Appearance Yellow gas Density 1.4 (air=1) Melting point -145 °C
Boiling point -23 °C
Structure Molecular shape linear C=N=N Dipole moment polar Hazards R-phrases R12 R19 R22 R66 R67 S-phrases S9 S16 S29 S33 Main hazards toxic and explosive Related compounds Related compounds R2CN2 R = Ph, tms, CF3  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions.[1]
Contents
Use
For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids into their methyl esters or into their homologues (see Arndt-Eistert synthesis). In the Buchner-Curtius-Schlotterbeck reaction (1885) diazomethane reacts with an aldehyde to form ketones. Diazomethane is also frequently used as a carbene source. It readily takes part in 1,3-dipolar cycloadditions.
Preparation
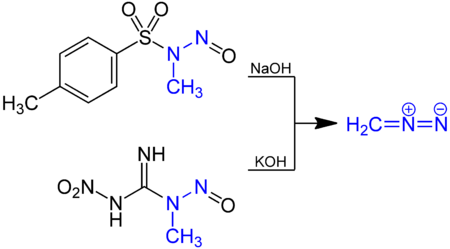
Diazomethane is prepared by hydrolysis of an ethereal solution of an N-methyl nitrosamide with aqueous base. The traditional precursor is N-Nitroso-N-methylurea, but this compound is somewhat unstable and nowadays such compounds as N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) are preferred.[2]
CH2N2 reacts with basic solutions of 2H2O to give the deuterated derivative C2H2N2.[3]
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of benzoic acid in cold Et2O. Unreacted benzoic acid is then back-titrated with standard NaOH. Alternatively, the concentration of CH2N2 in Et2O can be determined spectrophotometrically at 410 nm where its extinction coefficient, ε, is 7.2.[citation needed]
Related compounds
Many substituted derivatives of diazomethane have been prepared:
- The very stable (CF3)2CN2 (b.p. 12–13 °C),[4]
- Ph2CN2 (m.p. 29–30 °C).[5]
- (CH3)3SiCHN2 (trimethylsilyldiazomethane), which is commercially available as a solution and is as effective as CH2N2 for methylation.[6]
- PhC(H)N2, a red liquid b.p.< 25 °C at 0.1 mm Hg.[7]
Safety
Diazomethane is toxic by inhalation or by contact with the skin or eyes (TLV 0.2ppm). Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.[8] Symptoms may be delayed. Deaths from diazomethane poisoning have been reported. In one instance a laboratory worker consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.[9] Like any other alkylating agent it is expected to be carcinogenic, but such concerns are overshadowed by its serious acute toxicity.
CH2N2 may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware.[citation needed] Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
The compound explodes when heated beyond 100 °C.
References
- ^ L.D. Proctor, A.J. Warr, Org. Proc. Res. Dev., 2002, 6, 884–92. The authors report on a continuous process capable of generating 50-60 tons/year while the total inventory at any point in time is less than 80 g.
- ^ J. A. Moore; D. E. Reed (1973), "Diazomethane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0351; Coll. Vol. 5: 351
- ^ P. G. Gassman and W. J. Greenlee (1988), "Dideuterodiazomethane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0432; Coll. Vol. 6: 432
- ^ W. J. Middleton; D. M. Gale (1988), "Bis(Trifluoromethyl))diazomethane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0161; Coll. Vol. 6: 161
- ^ L. I. Smith, K. L. Howard (1955), "Diphenyldiazomethane”", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0351; Coll. Vol. 3: 351
- ^ T. Shioiri, T. Aoyama, S. Mori, "Trimethylsilyldiazomethane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0612; Coll. Vol. 8: 612
- ^ X. Creary (1990), "Tosylhydrazone Salt Pyrolyses: Phenydiazomethanes", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0438; Coll. Vol. 7: 438
- ^ Muir, GD (ed.) 1971, Hazards in the Chemical Laboratory, The Royal Institute of Chemistry, London.
- ^ LeWinn, E.B. "Diazomethane Poisoning: Report of a fatal case with autopsy", The American Journal of the Medical Sciences, 1949, 218, 556-562.
External links
- MSDS diazomethane
- Sigmaaldrich technical bulletin (PDF)
- Sigma-Aldrich diazomethane applications and commercial availability of (Diazald) precursor
- The Buchner-Curtius-Schlotterbeck reaction @ Institute of Chemistry, Skopje, Macedonia
Categories:- Diazo compounds
- Methylating agents
- IARC Group 3 carcinogens
Wikimedia Foundation. 2010.