- Reduction of nitro compounds
-
The chemical reactions described as reduction of nitro compounds can be facilitated by many different reagents and reaction conditions. Historically, the nitro group was one of the first functional groups to be reduced, due to the ease of nitro-group reduction.
Nitro-groups behave differently whether a neighboring hydrogen is present or not. Thus, reduction conditions can be initially classified by starting materials: aliphatic nitro compounds or aromatic nitro compounds. Secondary classifications are based upon reaction products.
Contents
Aliphatic nitro compounds
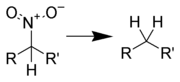
Reduction to hydrocarbons
Hydrodenitration (replacement of a nitro group with hydrogen) is difficult to achieve, but can be completed by catalytic hydrogenation over platinum on silica gel at high temperatures.[1]
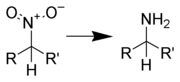
Reduction to amines
Aliphatic nitro compounds can be reduced to aliphatic amines using several different reagents:
- Catalytic hydrogenation using platinum(IV) oxide (PtO2)[2] or Raney nickel[3]
- Iron metal in refluxing acetic acid[4]
- Samarium diiodide[5]
α,β-Unsaturated nitro compounds can be reduced to saturated amines using:
- Catalytic hydrogenation over palladium-on-carbon
- Iron metal
- Lithium aluminium hydride[6] (Note: Hydroxylamine and oxime impurities are typically found.)
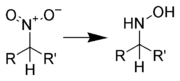
Reduction to hydroxylamines
Aliphatic nitro compounds can be reduced to aliphatic hydroxylamines using diborane.[7]
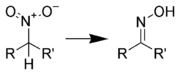
Reduction to oximes
Nitro compounds are typically reduced to oximes using metal salts, such as stannous chloride[8] or chromium(II) chloride.[9] Additionally, catalytic hydrogenation using a controlled amount of hydrogen can generate oximes.[10]
Aromatic nitro compounds
The reduction of aryl nitro compounds can be finely tuned to obtain a different products typically in high yields.
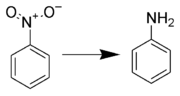
Reduction to anilines
Many methods for the production of anilines from aryl nitro compounds exist, such as:
- Catalytic hydrogenation using palladium-on-carbon,[11] platinum(IV) oxide, or Raney nickel[12]
- Iron in acidic media[13] (Note: Iron is particularly well suited for this reduction as the reaction conditions are typically gentle and also because iron has a high functional group tolerance.) (See Bechamp reduction)
- Sodium hydrosulfite[14]
- Sodium sulfide (or hydrogen sulfide and base)
- Tin(II) chloride
- Titanium(III) chloride
- Zinc
- Samarium[15]
It is also possible to form a nitroaniline by reduction of a dinitroarene using sodium sulfide.[16]
Metal hydrides are typically not used to reduce aryl nitro compounds to anilines because they tend to produce azo compounds. (See below)
Reduction to hydroxylamines
Several methods for the production of aryl hydroxylamines from aryl nitro compounds exist:
- Raney nickel and hydrazine at 0-10 °C[17]
- Electrolytic reduction[18]
- Zinc metal in aqueous ammonium chloride[19]
Reduction to hydrazo compounds
Treatment of nitroarenes with excess zinc metal results in the formation of N,N'-diarylhydrazine.[20]
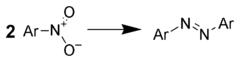
Reduction to azo compounds
Treatment of aromatic nitro compounds with metal hydrides gives good yields of azo compounds. For example, one could use:
- Lithium aluminium hydride[21]
- Zinc metal with sodium hydroxide.[20] (Excess zinc will reduce the azo group to a hydrazino compound.)
Reduction to azoxy compounds
References
- ^ M. J. Guttieri and W. F. Maier (1984). "Selective cleavage of carbon-nitrogen bonds with platinum". J. Org. Chem. 49 (16): 2875–2880. doi:10.1021/jo00190a006.
- ^ A. T. Nielsen (1962). "The Isomeric Dinitrocyclohexanes. II. Stereochemistry". J. Org. Chem. 27 (6): 1998–2001. doi:10.1021/jo01053a019.
- ^ Dauben, Jr., H. J.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, Jr., A. G. (1963), "Cycloheptanone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0221; Coll. Vol. 4: 221
- ^ Senkus, M. (1948). Ind. Eng. Chem.. 40. pp. 506.
- ^ A. S. Kende and J. S. Mendoza (1991). "Controlled reduction of nitroalkanes to alkyl hydroxylamines or amines by samarium diiodide". Tetrahedron Letters 32 (14): 1699–1702. doi:10.1016/S0040-4039(00)74307-3.
- ^ A. Burger, M. L. Stein and J. B. Clements (1957). "Some Pyridylnitroalkenes, Nitroalkanols, and Alkylamines". J. Org. Chem. 22 (2): 143–144. doi:10.1021/jo01353a010.
- ^ H. Feuer, R. S. Bartlett, B. F. Vincent and R. S. Anderson (1965). "Diborane Reduction of Nitro Salts. A New Synthesis of N-Monosubstituted Hydroxylamines". J. Org. Chem. 30 (9): 2880–2882. doi:10.1021/jo01020a002.
- ^ Braun, V. J.; Sobecki, W. (1911). "Über primäre Dinitro-, Nitronitrit- und Dialdoxim-Verbindungen der Fettreihe". Ber. 44 (3): 2526–2534. doi:10.1002/cber.19110440377.
- ^ J. R. Hanson and E. Premuzic (1967). "Applications of chromous chloride--II : The reduction of some steroidal nitro-compounds". Tetrahedron 23 (10): 4105–4110. doi:10.1016/S0040-4020(01)97921-9.
- ^ C. Grundmann (1950). "Über die partielle Reduktion von Nitro-cyclohexan". Angewandte Chemie 62 (23-24): 558–560. doi:10.1002/ange.19500622304.
- ^ Bavin, P. M. G. (1973), "2-Aminofluorene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0030; Coll. Vol. 5: 30
- ^ Allen, C. F. H.; VanAllan, J. (1955)), "2-Amino-p-cymene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0063; Coll. Vol. 3: 63
- ^ Fox, B. A.; Threlfall, T. L. (1973), "2,3-Diaminopyridine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0346; Coll. Vol. 5: 346
- ^ Redemann, C. T.; Redemann, C. E. (1955), "5-Amino-2,3-dihydro-1,4-phthalazinedione", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0069; Coll. Vol. 3: 69
- ^ Basu, M. K. (2000). "Ultrasound-promoted highly efficient reduction of aromatic nitro compounds to the aromatic amines by samarium/ammonium chloride". Tet. Lett. 41 (30): 5603. doi:10.1016/S0040-4039(00)00917-5.
- ^ Hartman, W. W.; Silloway, H. L. (1955), "2-Amino-4-nitrophenol", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0082; Coll. Vol. 3: 82
- ^ Ayyangar, N. R.; Brahme, K. C.; Kalkote, U. R.; Srinivasan, K. V. (1984). "Facile Transfer-Reduction of Nitroarenes to N Arylhydroxylamines with Hydrazine in the Presence of Raney Nickel". Synthesis 1984 (11): 938. doi:10.1055/s-1984-31027.
- ^ Harman, R. E. (1963), "Chloro-p-benzoquinone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0148; Coll. Vol. 4: 148
- ^ Kamm, O. (1941), "β-Phenylhydroxylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0445; Coll. Vol. 1: 445
- ^ a b Bigelow, H. E.; Robinson, D. B. (1955), "Azobenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0103; Coll. Vol. 3: 103
- ^ R. F. Nystrom and W. G. Brown (1948). "Reduction of Organic Compounds by Lithium Aluminum Hydride. III. Halides, Quinones, Miscellaneous Nitrogen Compounds". J. Am. Chem. Soc. 70 (11): 3738–3740. doi:10.1021/ja01191a057.
Categories:- Organic redox reactions
Wikimedia Foundation. 2010.