- Cyclin-dependent kinase 2
-
"CDK2" redirects here. For the airport with the Transport Canada identifier CDK2, see Diavik Airport.
Cyclin-dependent kinase 2 also known as cell division protein kinase 2 is an enzyme that in humans is encoded by the CDK2 gene.[1][2]
Contents
Function
The protein encoded by this gene is a member of the cyclin-dependent kinase family of Ser/Thr protein kinases. This protein kinase is highly similar to the gene products of S. cerevisiae cdc28, and S. pombe cdc2, also known as Cdk1 in humans. It is a catalytic subunit of the cyclin-dependent kinase complex, whose activity is restricted to the G1-S phase of the cell cycle, and is essential for the G1/S transition. This protein associates with and is regulated by the regulatory subunits of the complex including cyclin E or A. Cyclin E binds G1 phase Cdk2, which is required for the transition from G1 to S phase while binding with Cyclin A is required to progress through the S phase. Its activity is also regulated by phosphorylation. Two alternatively spliced variants and multiple transcription initiation sites of this gene have been reported.[2]
The role of this protein in G1-S transition has been recently questioned as cells lacking Cdk2 are reported to have no problem during this transition.[3]
Inhibitors
Known CDK inhibitors are p21Cip1 (CDKN1A) and p27Kip1 (CDKN1B).[4] Drugs that inhibit Cdk2 and arrest the cell cycle, such as GW8510, may reduce the sensitivity of the epithelium to many cell cycle-active antitumor agents and, therefore, represent a strategy for prevention of chemotherapy-induced alopecia.[5]
Gene regulation
In melanocytic cell types, expression of the CDK2 gene is regulated by the Microphthalmia-associated transcription factor.[6][7]
Interactions
Cyclin-dependent kinase 2 has been shown to interact with Retinoblastoma-like protein 2,[8][9] Retinoblastoma-like protein 1,[8][10] Flap structure-specific endonuclease 1,[11] CEBPA,[12] BRCA1,[13][14][15] PPM1B,[16] Cyclin A1,[17][18][19][20] Cyclin E1,[8][21][22][23][24][25] SKP2,[26][27][28] PPP2CA,[16] ORC1L,[29] CDK2AP1,[30] CDKN3,[31][32][33] CDKN1B[24][26][34][35][36] and P21.[21][28][33][36][37]
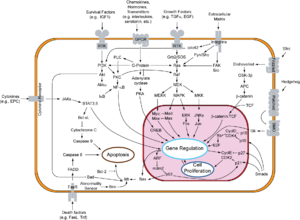 Overview of signal transduction pathways involved in apoptosis.
Overview of signal transduction pathways involved in apoptosis.
References
- ^ Tsai LH, Harlow E, Meyerson M (September 1991). "Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase". Nature 353 (6340): 174–7. doi:10.1038/353174a0. PMID 1653904.
- ^ a b "Entrez Gene: CDK2 cyclin-dependent kinase 2". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1017.
- ^ Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P (October 2003). "Cdk2 knockout mice are viable". Curr. Biol. 13 (20): 1775–85. doi:10.1016/j.cub.2003.09.024. PMID 14561402. http://linkinghub.elsevier.com/retrieve/pii/S0960982203006936.
- ^ Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R (March 1998). "Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade". Mol. Cell 1 (4): 553–63. doi:10.1016/S1097-2765(00)80055-6. PMID 9660939.
- ^ Davis ST, Benson BG, Bramson HN, Chapman DE, Dickerson SH, Dold KM, Eberwein DJ, Edelstein M, Frye SV, Gampe Jr RT, Griffin RJ, Harris PA, Hassell AM, Holmes WD, Hunter RN, Knick VB, Lackey K, Lovejoy B, Luzzio MJ, Murray D, Parker P, Rocque WJ, Shewchuk L, Veal JM, Walker DH, Kuyper LF (January 2001). "Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors". Science 291 (5501): 134–7. doi:10.1126/science.291.5501.134. PMID 11141566.
- ^ Du J, Widlund HR, Horstmann MA, et al. (2004). "Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF". Cancer Cell 6 (6): 565–76. doi:10.1016/j.ccr.2004.10.014. PMID 15607961.
- ^ Hoek KS, Schlegel NC, Eichhoff OM, et al. (2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell Melanoma Res. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ^ a b c Shanahan, F; Seghezzi W, Parry D, Mahony D, Lees E (Feb. 1999). "Cyclin E Associates with BAF155 and BRG1, Components of the Mammalian SWI-SNF Complex, and Alters the Ability of BRG1 To Induce Growth Arrest". Mol. Cell. Biol. (UNITED STATES) 19 (2): 1460–9. ISSN 0270-7306. PMC 116074. PMID 9891079. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=116074.
- ^ Lacy, S; Whyte P (May. 1997). "Identification of a p130 domain mediating interactions with cyclin A/cdk 2 and cyclin E/cdk 2 complexes". Oncogene (ENGLAND) 14 (20): 2395–406. doi:10.1038/sj.onc.1201085. ISSN 0950-9232. PMID 9188854.
- ^ Leng, Xiaohong; Noble Martin, Adams Peter D, Qin Jun, Harper J Wade (Apr. 2002). "Reversal of Growth Suppression by p107 via Direct Phosphorylation by Cyclin D1/Cyclin-Dependent Kinase 4". Mol. Cell. Biol. (United States) 22 (7): 2242–54. doi:10.1128/MCB.22.7.2242-2254.2002. ISSN 0270-7306. PMC 133692. PMID 11884610. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=133692.
- ^ Henneke, Ghislaine; Koundrioukoff Stéphane, Hübscher Ulrich (Jul. 2003). "Phosphorylation of human Fen1 by cyclin-dependent kinase modulates its role in replication fork regulation". Oncogene (England) 22 (28): 4301–13. doi:10.1038/sj.onc.1206606. ISSN 0950-9232. PMID 12853968.
- ^ Wang, H; Iakova P, Wilde M, Welm A, Goode T, Roesler W J, Timchenko N A (Oct. 2001). "C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4". Mol. Cell (United States) 8 (4): 817–28. doi:10.1016/S1097-2765(01)00366-5. ISSN 1097-2765. PMID 11684017.
- ^ Chen, Y; Farmer A A, Chen C F, Jones D C, Chen P L, Lee W H (Jul. 1996). "BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner". Cancer Res. (UNITED STATES) 56 (14): 3168–72. ISSN 0008-5472. PMID 8764100.
- ^ Ruffner, H; Jiang W, Craig A G, Hunter T, Verma I M (Jul. 1999). "BRCA1 Is Phosphorylated at Serine 1497 In Vivo at a Cyclin-Dependent Kinase 2 Phosphorylation Site". Mol. Cell. Biol. (UNITED STATES) 19 (7): 4843–54. ISSN 0270-7306. PMC 84283. PMID 10373534. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=84283.
- ^ Wang, H; Shao N, Ding Q M, Cui J, Reddy E S, Rao V N (Jul. 1997). "BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases". Oncogene (ENGLAND) 15 (2): 143–57. doi:10.1038/sj.onc.1201252. ISSN 0950-9232. PMID 9244350.
- ^ a b Cheng, A; Kaldis P, Solomon M J (Nov. 2000). "Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms". J. Biol. Chem. (UNITED STATES) 275 (44): 34744–9. doi:10.1074/jbc.M006210200. ISSN 0021-9258. PMID 10934208.
- ^ Sweeney, C; Murphy M, Kubelka M, Ravnik S E, Hawkins C F, Wolgemuth D J, Carrington M (Jan. 1996). "A distinct cyclin A is expressed in germ cells in the mouse". Development (ENGLAND) 122 (1): 53–64. ISSN 0950-1991. PMID 8565853.
- ^ Yang, R; Morosetti R, Koeffler H P (Mar. 1997). "Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines". Cancer Res. (UNITED STATES) 57 (5): 913–20. ISSN 0008-5472. PMID 9041194.
- ^ Müller-Tidow, C; Wang W, Idos G E, Diederichs S, Yang R, Readhead C, Berdel W E, Serve H, Saville M, Watson R, Koeffler H P (Apr. 2001). "Cyclin A1 directly interacts with B-myb and cyclin A1/cdk2 phosphorylate B-myb at functionally important serine and threonine residues: tissue-specific regulation of B-myb function". Blood (United States) 97 (7): 2091–7. doi:10.1182/blood.V97.7.2091. ISSN 0006-4971. PMID 11264176.
- ^ Brown, N R; Noble M E, Endicott J A, Johnson L N (Nov. 1999). "The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases". Nat. Cell Biol. (England) 1 (7): 438–43. doi:10.1038/15674. ISSN 1465-7392. PMID 10559988.
- ^ a b McKenzie, Pamela P; Danks Mary K, Kriwacki Richard W, Harris Linda C (Jul. 2003). "P21Waf1/Cip1 dysfunction in neuroblastoma: a novel mechanism of attenuating G0-G1 cell cycle arrest". Cancer Res. (United States) 63 (13): 3840–4. ISSN 0008-5472. PMID 12839982.
- ^ Koff, A; Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza B R, Roberts J M (Sep. 1992). "Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle". Science (UNITED STATES) 257 (5077): 1689–94. doi:10.1126/science.1388288. ISSN 0036-8075. PMID 1388288.
- ^ Mayer, Christine; Zhao Jian, Yuan Xuejun, Grummt Ingrid (Feb. 2004). "mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability". Genes Dev. (United States) 18 (4): 423–34. doi:10.1101/gad.285504. ISSN 0890-9369. PMC 359396. PMID 15004009. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=359396.
- ^ a b Connor, Michael K; Kotchetkov Rouslan, Cariou Sandrine, Resch Ansgar, Lupetti Rafaella, Beniston Richard G, Melchior Frauke, Hengst Ludger, Slingerland Joyce M (Jan. 2003). "CRM1/Ran-Mediated Nuclear Export of p27Kip1 Involves a Nuclear Export Signal and Links p27 Export and Proteolysis". Mol. Biol. Cell (United States) 14 (1): 201–13. doi:10.1091/mbc.E02-06-0319. ISSN 1059-1524. PMC 140238. PMID 12529437. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140238.
- ^ Boudrez, A; Beullens M, Groenen P, Van Eynde A, Vulsteke V, Jagiello I, Murray M, Krainer A R, Stalmans W, Bollen M (Aug. 2000). "NIPP1-mediated interaction of protein phosphatase-1 with CDC5L, a regulator of pre-mRNA splicing and mitotic entry". J. Biol. Chem. (UNITED STATES) 275 (33): 25411–7. doi:10.1074/jbc.M001676200. ISSN 0021-9258. PMID 10827081.
- ^ a b Rosner, Margit; Hengstschläger Markus (Nov. 2004). "Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2". J. Biol. Chem. (United States) 279 (47): 48707–15. doi:10.1074/jbc.M405528200. ISSN 0021-9258. PMID 15355997.
- ^ Marti, A; Wirbelauer C, Scheffner M, Krek W (May. 1999). "Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation". Nat. Cell Biol. (ENGLAND) 1 (1): 14–9. doi:10.1038/8984. ISSN 1465-7392. PMID 10559858.
- ^ a b Yam, C H; Ng R W, Siu W Y, Lau A W, Poon R Y (Jan. 1999). "Regulation of Cyclin A-Cdk2 by SCF Component Skp1 and F-Box Protein Skp2". Mol. Cell. Biol. (UNITED STATES) 19 (1): 635–45. ISSN 0270-7306. PMC 83921. PMID 9858587. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=83921.
- ^ Méndez, Juan; Zou-Yang X Helena, Kim So-Young, Hidaka Masumi, Tansey William P, Stillman Bruce (Mar. 2002). "Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication". Mol. Cell (United States) 9 (3): 481–91. doi:10.1016/S1097-2765(02)00467-7. ISSN 1097-2765. PMID 11931757.
- ^ Shintani, S; Ohyama H, Zhang X, McBride J, Matsuo K, Tsuji T, Hu M G, Hu G, Kohno Y, Lerman M, Todd R, Wong D T (Sep. 2000). "p12DOC-1 Is a Novel Cyclin-Dependent Kinase 2-Associated Protein". Mol. Cell. Biol. (UNITED STATES) 20 (17): 6300–7. doi:10.1128/MCB.20.17.6300-6307.2000. ISSN 0270-7306. PMC 86104. PMID 10938106. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86104.
- ^ Yeh, Chau-Ting; Lu Su-Chuan, Chao Chung-Hao, Chao Mei-Ling (May. 2003). "Abolishment of the interaction between cyclin-dependent kinase 2 and Cdk-associated protein phosphatase by a truncated KAP mutant". Biochem. Biophys. Res. Commun. (United States) 305 (2): 311–4. doi:10.1016/S0006-291X(03)00757-5. ISSN 0006-291X. PMID 12745075.
- ^ Hannon, G J; Casso D, Beach D (Mar. 1994). "KAP: a dual specificity phosphatase that interacts with cyclin-dependent kinases". Proc. Natl. Acad. Sci. U.S.A. (UNITED STATES) 91 (5): 1731–5. doi:10.1073/pnas.91.5.1731. ISSN 0027-8424. PMC 43237. PMID 8127873. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=43237.
- ^ a b Harper, J W; Adami G R, Wei N, Keyomarsi K, Elledge S J (Nov. 1993). "The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases". Cell (UNITED STATES) 75 (4): 805–16. doi:10.1016/0092-8674(93)90499-G. ISSN 0092-8674. PMID 8242751.
- ^ Youn, Cha-Kyung; Cho Hyun-Ju, Kim Soo-Hyun, Kim Hong-Beum, Kim Mi-Hwa, Chang In-Youb, Lee Jung-Sup, Chung Myung-Hee, Hahm Kyung-Soo, You Ho Jin (Feb. 2005). "Bcl-2 expression suppresses mismatch repair activity through inhibition of E2F transcriptional activity". Nat. Cell Biol. (England) 7 (2): 137–47. doi:10.1038/ncb1215. ISSN 1465-7392. PMID 15619620.
- ^ Porter, Lisa A; Kong-Beltran Monica, Donoghue Daniel J (Sep. 2003). "Spy1 Interacts with p27Kip1 to Allow G1/S Progression". Mol. Biol. Cell (United States) 14 (9): 3664–74. doi:10.1091/mbc.E02-12-0820. ISSN 1059-1524. PMC 196558. PMID 12972555. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=196558.
- ^ a b Law, Brian K; Chytil Anna, Dumont Nancy, Hamilton Elizabeth G, Waltner-Law Mary E, Aakre Mary E, Covington Cassondra, Moses Harold L (Dec. 2002). "Rapamycin Potentiates Transforming Growth Factor β-Induced Growth Arrest in Nontransformed, Oncogene-Transformed, and Human Cancer Cells". Mol. Cell. Biol. (United States) 22 (23): 8184–98. doi:10.1128/MCB.22.23.8184-8198.2002. ISSN 0270-7306. PMC 134072. PMID 12417722. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=134072.
- ^ Ono, T; Kitaura H, Ugai H, Murata T, Yokoyama K K, Iguchi-Ariga S M, Ariga H (Oct. 2000). "TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase". J. Biol. Chem. (UNITED STATES) 275 (40): 31145–54. doi:10.1074/jbc.M003031200. ISSN 0021-9258. PMID 10878006.
Further reading
- Kaldis P, Aleem E (2007). "Cell cycle sibling rivalry: Cdc2 vs. Cdk2". Cell Cycle 4 (11): 1491–4. doi:10.4161/cc.4.11.2124. PMID 16258277.
- Moore NL, Narayanan R, Weigel NL (2007). "CYCLIN DEPENDENT KINASE 2 AND THE REGULATION OF HUMAN PROGESTERONE RECEPTOR ACTIVITY". Steroids 72 (2): 202–9. doi:10.1016/j.steroids.2006.11.025. PMC 1950255. PMID 17207508. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1950255.
PDB gallery 1aq1: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR STAUROSPORINE1b38: HUMAN CYCLIN-DEPENDENT KINASE 21b39: HUMAN CYCLIN-DEPENDENT KINASE 2 PHOSPHORYLATED ON THR 1601buh: CRYSTAL STRUCTURE OF THE HUMAN CDK2 KINASE COMPLEX WITH CELL CYCLE-REGULATORY PROTEIN CKSHS11ckp: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR PURVALANOL B1di8: THE STRUCTURE OF CYCLIN-DEPENDENT KINASE 2 (CDK2) IN COMPLEX WITH 4-[3-HYDROXYANILINO]-6,7-DIMETHOXYQUINAZOLINE1dm2: HUMAN CYCLIN-DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR HYMENIALDISINE1e1v: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR NU20581e1x: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR NU60271e9h: THR 160 PHOSPHORYLATED CDK2 - HUMAN CYCLIN A3 COMPLEX WITH THE INHIBITOR INDIRUBIN-5-SULPHONATE BOUND1f5q: CRYSTAL STRUCTURE OF MURINE GAMMA HERPESVIRUS CYCLIN COMPLEXED TO HUMAN CYCLIN DEPENDENT KINASE 21fin: CYCLIN A-CYCLIN-DEPENDENT KINASE 2 COMPLEX1fq1: CRYSTAL STRUCTURE OF KINASE ASSOCIATED PHOSPHATASE (KAP) IN COMPLEX WITH PHOSPHO-CDK21fvt: THE STRUCTURE OF CYCLIN-DEPENDENT KINASE 2 (CDK2) IN COMPLEX WITH AN OXINDOLE INHIBITOR1fvv: THE STRUCTURE OF CDK2/CYCLIN A IN COMPLEX WITH AN OXINDOLE INHIBITOR1g5s: CRYSTAL STRUCTURE OF HUMAN CYCLIN DEPENDENT KINASE 2 (CDK2) IN COMPLEX WITH THE INHIBITOR H7171gih: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE CDK4 INHIBITOR1gii: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE CDK4 INHIBITOR1gij: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE CDK4 INHIBITOR1gy3: PCDK2/CYCLIN A IN COMPLEX WITH MGADP, NITRATE AND PEPTIDE SUBSTRATE1gz8: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 2-AMINO-6-(3'-METHYL-2'-OXO)BUTOXYPURINE1h00: CDK2 IN COMPLEX WITH A DISUBSTITUTED 4, 6-BIS ANILINO PYRIMIDINE CDK4 INHIBITOR1h07: CDK2 IN COMPLEX WITH A DISUBSTITUTED 4, 6-BIS ANILINO PYRIMIDINE CDK4 INHIBITOR1h08: CDK2 IN COMPLEX WITH A DISUBSTITUTED 2, 4-BIS ANILINO PYRIMIDINE CDK4 INHIBITOR1h0v: HUMAN CYCLIN DEPENDENT PROTEIN KINASE 2 IN COMPLEX WITH THE INHIBITOR 2-AMINO-6-[(R)-PYRROLIDINO-5'-YL]METHOXYPURINE1h0w: HUMAN CYCLIN DEPENDENT PROTEIN KINASE 2 IN COMPLEX WITH THE INHIBITOR 2-AMINO-6-[CYCLOHEX-3-ENYL]METHOXYPURINE1h1p: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH THE INHIBITOR NU20581h1q: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH THE INHIBITOR NU60941h1r: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH THE INHIBITOR NU60861h1s: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH THE INHIBITOR NU61021h24: CDK2/CYCLIN A IN COMPLEX WITH A 9 RESIDUE RECRUITMENT PEPTIDE FROM E2F1h25: CDK2/CYCLIN A IN COMPLEX WITH AN 11-RESIDUE RECRUITMENT PEPTIDE FROM RETINOBLASTOMA-ASSOCIATED PROTEIN1h26: CDK2/CYCLIN A IN COMPLEX WITH AN 11-RESIDUE RECRUITMENT PEPTIDE FROM P531h27: CDK2/CYCLIN A IN COMPLEX WITH AN 11-RESIDUE RECRUITMENT PEPTIDE FROM P271h28: CDK2/CYCLIN A IN COMPLEX WITH AN 11-RESIDUE RECRUITMENT PEPTIDE FROM P1071hck: HUMAN CYCLIN-DEPENDENT KINASE 21hcl: HUMAN CYCLIN-DEPENDENT KINASE 21jst: PHOSPHORYLATED CYCLIN-DEPENDENT KINASE-2 BOUND TO CYCLIN A1jsu: P27(KIP1)/CYCLIN A/CDK2 COMPLEX1jsv: The structure of cyclin-dependent kinase 2 (CDK2) in complex with 4-[(6-amino-4-pyrimidinyl)amino]benzenesulfonamide1jvp: Crystal structure of human CDK2 (unphosphorylated) in complex with PKF049-3651ke5: CDK2 complexed with N-methyl-4-{[(2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]amino}benzenesulfonamide1ke6: CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH N-METHYL-{4-[2-(7-OXO-6,7-DIHYDRO-8H-[1,3]THIAZOLO[5,4-E]INDOL-8-YLIDENE)HYDRAZINO]PHENYL}METHANESULFONAMIDE1ke7: CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 3-{[(2,2-DIOXIDO-1,3-DIHYDRO-2-BENZOTHIEN-5-YL)AMINO]METHYLENE}-5-(1,3-OXAZOL-5-YL)-1,3-DIHYDRO-2H-INDOL-2-ONE1ke8: CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 4-{[(2-OXO-1,2-DIHYDRO-3H-INDOL-3-YLIDENE)METHYL]AMINO}-N-(1,3-THIAZOL-2-YL)BENZENESULFONAMIDE1ke9: CYCLIN-DEPENDENT KINASE 2 (CDK2) COMPLEXED WITH 3-{[4-({[AMINO(IMINO)METHYL]AMINOSULFONYL)ANILINO]METHYLENE}-2-OXO-2,3-DIHYDRO-1H-INDOLE1ogu: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH A 2-ARYLAMINO-4-CYCLOHEXYLMETHYL-5-NITROSO-6-AMINOPYRIMIDINE INHIBITOR1oi9: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH A 6-CYCLOHEXYLMETHYLOXY-2-ANILINO-PURINE INHIBITOR1oiq: IMIDAZOPYRIDINES: A POTENT AND SELECTIVE CLASS OF CYCLIN-DEPENDENT KINASE INHIBITORS IDENTIFIED THROUGH STRUCTURE-BASED HYBRIDISATION1oir: IMIDAZOPYRIDINES: A POTENT AND SELECTIVE CLASS OF CYCLIN-DEPENDENT KINASE INHIBITORS IDENTIFIED THROUGH STRUCTURE-BASED HYBRIDISATION1oit: IMIDAZOPYRIDINES: A POTENT AND SELECTIVE CLASS OF CYCLIN-DEPENDENT KINASE INHIBITORS IDENTIFIED THROUGH STRUCTURE-BASED HYBRIDISATION1oiu: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH A 6-CYCLOHEXYLMETHYLOXY-2-ANILINO-PURINE INHIBITOR1oiy: STRUCTURE OF HUMAN THR160-PHOSPHO CDK2/CYCLIN A COMPLEXED WITH A 6-CYCLOHEXYLMETHYLOXY-2-ANILINO-PURINE INHIBITOR1okv: CYCLIN A BINDING GROOVE INHIBITOR H-ARG-ARG-LEU-ILE-PHE-NH21okw: CYCLIN A BINDING GROOVE INHIBITOR AC-ARG-ARG-LEU-ASN-(M-CL-PHE)-NH21ol1: CYCLIN A BINDING GROOVE INHIBITOR H-CIT-CIT-LEU-ILE-(P-F-PHE)-NH21ol2: CYCLIN A BINDING GROOVE INHIBITOR H-ARG-ARG-LEU-ASN-(P-F-PHE)-NH21p2a: The structure of cyclin dependent kinase 2 (CKD2) with a trisubstituted naphthostyril inhibitor1p5e: The strucure of phospho-CDK2/cyclin A in complex with the inhibitor 4,5,6,7-tetrabromobenzotriazole (TBS)1pf8: Crystal Structure of Human Cyclin-Dependent Kinase 2 Complexed with a Nucleoside Inhibitor1pkd: THE CRYSTAL STRUCTURE OF UCN-01 IN COMPLEX WITH PHOSPHO-CDK2/CYCLIN A1pw2: APO STRUCTURE OF HUMAN CYCLIN-DEPENDENT KINASE 21pxi: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-(2,5-Dichloro-thiophen-3-yl)-pyrimidin-2-ylamine1pxj: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-ylamine1pxk: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR N-[4-(2,4-Dimethyl-thiazol-5-yl)pyrimidin-2-yl]-N'-hydroxyiminoformamide1pxl: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR [4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-amine1pxm: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 3-[4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-ylamino]-phenol1pxn: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR 4-[4-(4-Methyl-2-methylamino-thiazol-5-yl)-pyrimidin-2-ylamino]-phenol1pxo: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR [4-(2-Amino-4-methyl-thiazol-5-yl)-pyrimidin-2-yl]-(3-nitro-phenyl)-amine1pxp: HUMAN CYCLIN DEPENDENT KINASE 2 COMPLEXED WITH THE INHIBITOR N-[4-(2,4-Dimethyl-thiazol-5-yl)-pyrimidin-2-yl]-N',N'-dimethyl-benzene-1,4-diamine1pye: Crystal structure of CDK2 with inhibitor1qmz: PHOSPHORYLATED CDK2-CYCLYIN A-SUBSTRATE PEPTIDE COMPLEX1r78: CDK2 complex with a 4-alkynyl oxindole inhibitor1urc: CYCLIN A BINDING GROOVE INHIBITOR ACE-ARG-LYS-LEU-PHE-GLY1urw: CDK2 IN COMPLEX WITH AN IMIDAZO[1,2-B]PYRIDAZINE1v1k: CDK2 IN COMPLEX WITH A DISUBSTITUTED 4, 6-BIS ANILINO PYRIMIDINE CDK4 INHIBITOR1vyw: STRUCTURE OF CDK2/CYCLIN A WITH PNU-2921371vyz: STRUCTURE OF CDK2 COMPLEXED WITH PNU-1812271w0x: CRYSTALS STRUCTURE OF HUMAN CDK2 IN COMPLEX WITH THE INHIBITOR OLOMOUCINE.1w8c: CO-CRYSTAL STRUCTURE OF 6-CYCLOHEXYLMETHOXY-8-ISOPROPYL-9H-PURIN-2-YLAMINE AND MONOMERIC CDK21w98: THE STRUCTURAL BASIS OF CDK2 ACTIVATION BY CYCLIN E1wcc: SCREENING FOR FRAGMENT BINDING BY X-RAY CRYSTALLOGRAPHY1y8y: Crystal structure of human CDK2 complexed with a pyrazolo[1,5-a]pyrimidine inhibitor1y91: Crystal structure of human CDK2 complexed with a pyrazolo[1,5-a]pyrimidine inhibitor1ykr: Crystal structure of cdk2 with an aminoimidazo pyridine inhibitor2a0c: Human CDK2 in complex with olomoucine II, a novel 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitor2a4l: Human cyclin-dependent kinase 2 in complex with roscovitine2b52: Human cyclin dependent kinase 2 (CDK2) complexed with DPH-0425622b53: Human cyclin dependent kinase 2 (CDK2) complexed with DIN-2343252b54: Human cyclin dependent kinase 2 (CKD2)complexed with DIN-2323052b55: Human cyclin dependent kinase 2 (cdk2) complexed with indenopyraxole DIN-1013122bhe: HUMAN CYCLIN DEPENDENT PROTEIN KINASE 2 IN COMPLEX WITH THE INHIBITOR 5-BROMO-INDIRUBINE2bhh: HUMAN CYCLIN DEPENDENT PROTEIN KINASE 2 IN COMPLEX WITH THE INHIBITOR 4-HYDROXYPIPERINDINESULFONYL-INDIRUBINE2bkz: STRUCTURE OF CDK2-CYCLIN A WITH PHA-4046112bpm: STRUCTURE OF CDK2-CYCLIN A WITH PHA-6305292btr: STRUCTURE OF CDK2 COMPLEXED WITH PNU-1988732bts: STRUCTURE OF CDK2 COMPLEXED WITH PNU-2300322c4g: STRUCTURE OF CDK2-CYCLIN A WITH PHA-5335142c5n: DIFFERENTIAL BINDING OF INHIBITORS TO ACTIVE AND INACTIVE CDK2 PROVIDES INSIGHTS FOR DRUG DESIGN2c5o: DIFFERENTIAL BINDING OF INHIBITORS TO ACTIVE AND INACTIVE CDK2 PROVIDES INSIGHTS FOR DRUG DESIGNThe maximum number of images (100) is exceeded ! External links
Cell cycle proteins Cyclin CDK CDK inhibitor P53 p63 p73 family Phases and
checkpointsOther cellular phasesCategories:- Human proteins
- Cell cycle
- Proteins
Wikimedia Foundation. 2010.