- Direct Xa inhibitor
-
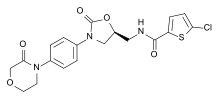 Rivaroxaban, the first synthetic direct Xa inhibitor marketed as a drug
Rivaroxaban, the first synthetic direct Xa inhibitor marketed as a drug
Direct factor Xa inhibitors ('xabans') are a class of anticoagulant drugs which act directly upon Factor X in the coagulation cascade, without using antithrombin as a mediator.[1]
Contents
Clinical uses
Direct factor Xa inhibitors are being used clinically. Clinical trials published in journals such as the New England Journal of Medicine and The Lancet have shown promise for these compounds as substitutes for the currently administered vitamin K antagonists or low molecular weight heparin. Advantages of orally administered direct Xa inhibitors lie in the fact that they have a predictable effect, do not require frequent monitoring or re-dosing, are given through the mouth and not by injection and have few (known) drug interactions. Disadvantages include the currently limited prospective experience and the theoretical interactions with statin medication, as they are metabolized at least in part by the same cytochrome enzyme, CYP3A4.
Examples
A naturally occurring inhibitor of factor Xa was first reported in 1987. Tuszynski et al. discovered antistasin, which was isolated from the extracts of Mexican leach, Haementeria officinalis. Soon after this, another naturally occurring inhibitor, tick anticoagulant peptide (TAP) was isolated from the extract of tick Ornithodoros moubata.
Examples include:
- Apixaban[2]
- Edoxaban
- Otamixaban[3][4]
- Rivaroxaban[5]
- YM466[6]
References
- ^ "Medscape.com". http://www.medscape.com/viewarticle/456874_5. Retrieved 2009-01-23.
- ^ Turpie AG (June 2007). "Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases". Arterioscler. Thromb. Vasc. Biol. 27 (6): 1238–47. doi:10.1161/ATVBAHA.107.139402. PMID 17379841. http://atvb.ahajournals.org/cgi/pmidlookup?view=long&pmid=17379841.
- ^ Cohen M, Bhatt DL, Alexander JH, et al. (May 2007). "Randomized, double-blind, dose-ranging study of otamixaban, a novel, parenteral, short-acting direct factor Xa inhibitor, in percutaneous coronary intervention: the SEPIA-PCI trial". Circulation 115 (20): 2642–51. doi:10.1161/CIRCULATIONAHA.106.653428. PMID 17502577. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=17502577.
- ^ Paccaly A, Ozoux ML, Chu V, et al. (December 2005). "Pharmacodynamic markers in the early clinical assessment of otamixaban, a direct factor Xa inhibitor". Thromb. Haemost. 94 (6): 1156–63. doi:10.1160/TH05-05-0347. PMID 16411387. http://www.schattauer.de/index.php?id=1268&pii=th05121156&no_cache=1.
- ^ Eriksson BI, Borris LC, Dahl OE, et al. (November 2006). "A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement". Circulation 114 (22): 2374–81. doi:10.1161/CIRCULATIONAHA.106.642074. PMID 17116766. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=17116766.
- ^ Iwatsuki Y, Sakata C, Kawasaki T, Okada M (October 2007). "Experimental model of lower limb ischemia in rats and the effect of YM466, an oral direct factor Xa inhibitor". Biol. Pharm. Bull. 30 (10): 1874–7. doi:10.1248/bpb.30.1874. PMID 17917254. http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/30.1874?from=PubMed.
External links
- Direct Factor Xa Inhibitors as Anticoagulants | PharmaXChange.info – A review and presentation on the available factor Xa inhibitors which can be used as anticoagulants.
- Oral Xa inhibitors – Literature review of the evidence regarding oral Xa inhibitors
This drug article relating to the blood and blood forming organs is a stub. You can help Wikipedia by expanding it.