- Organoarsenic chemistry
-
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compound is arsine. Despite their toxicity, organoarsenic biomolecules are well known.
Contents
History
Surprising for an area now considered of minor importance, organoarsenic chemistry played a prominent role in the history of the field of chemistry. The oldest known organoarsenic compound, the foul smelling cacodyl was reported in "cacodyl" (1760) and is sometimes classified as the first synthetic organometallic compound. The compound Salvarsan was one of the first pharmaceuticals, earning a Nobel prize for Paul Ehrlich.
Synthesis and classification
Arsenic typically occurs in the oxidation states (III) and (V), illustrated by the halides AsX3 (X = F, Cl, Br, I) and AsF5. Correspondingly, organoarsenic compounds are commonly found in these two oxidation states.[1]
Organoarsenic(V) chemistry and uses
Arsenic(V) compounds typically feature the functional groups RAsO(OH)2 or R2AsO(OH) (R = alkyl or aryl). Cacodylic acid, with the formula (CH3)2AsO2H, figures prominently throughout the chemistry of organoarsenic compounds. In contrast, the dimethylphosphonic acid is less significant in the corresponding chemistry of phosphorus. Cacodylic acid arises from the methylation of arsenic(III) oxide. Phenylarsonic acids can be accessed by the reaction of arsenic acid with anilines, the so-called Bechamp reaction.
The monomethylated acid, methanearsonic acid (CH3AsO(OH)2), is a precursor to fungicides (tradename Neoasozin) in the cultivation of rice and cotton. Derivatives of phenylarsonic acid (C6H5AsO(OH)2) are used as feed additives for livestock, including 4-hydroxy-3-nitrobenzenearsonic acid (3-NHPAA or Roxarsone), ureidophenylarsonic acid, and p-arsanilic acid. These applications are controversial as they introduce soluble forms of arsenic into the environment.
Compounds of arsenic(V) containing only organic ligands are rare, the pre-eminent member being the pentaphenyl derivative As(C6H5)5.[2]
Organoarsenic(III) chemistry and uses
Most such compounds are prepared by alkylation of AsCl3 and its derivatives using organolithium and Grignard reagents.[2] For example, the series trimethylarsine ((CH3)3As), dimethylarsenic chloride ((CH3)2AsCl), and methylarsenic dichloride (CH3AsCl2) is known. Reduction of the chloride derivatives with hydride reducing reagents affords the corresponding hydrides, such as dimethylarsine ((CH3)2AsH) and methylarsine (CH3AsH2). Similar manipulations apply to other organoarsenic chloride compounds.
An important route route to dimethylarsenic compounds begin with reduction of cacodylic acid (see above):
- (CH3)2AsO2H + 2 Zn + 4 HCl → (CH3)2AsH + 2 ZnCl2 + 2 H2O
- (CH3)2AsO2H + SO2 + HI → (CH3)2AsI + SO3 + H2O
A variety of heterocycles containing arsenic(III) are known. These include arsole, the arsenic analogue of pyrrole, and arsabenzene, the arsenic analogue of pyridine.
Symmetrical organoarsenic(III) compounds, e.g. trimethylarsine and triphenylarsine, are commonly used as ligands in coordination chemistry. They behave like phosphine ligands, but are less basic. The diarsine C6H4(As(CH3)2)2, known as diars, is a chelating ligand. Thorin is an indicator for several metals.
Organoarsenic(I) compounds and uses
Least significant in terms of commercial uses and numbers are the organoarsenic(I) compounds. The anti-syphylic drugs Salvarsan and Neosalvarsan are representative of this class. These compounds typically feature three bonds to As, but only As-As single bonds. Compounds of arsenic(I) but containing As=As double bonds are rare.
Chemical warfare
Organoarsenic compounds, especially those featuring As-Cl bonds, have been used as chemical weapons, especially during World War I. Infamous examples include "Lewisite" (chlorovinyl-2-arsenic dichloride) and "Clark I" (chlorodiphenylarsine). Phenyldichloroarsine is another one.
Organoarsenic compounds in nature
As arsenic is toxic to most life forms and it occurs in elevated concentration in some areas several detoxification strategies have evolved. Inorganic arsenic and its compounds, upon entering the food chain, are progressively metabolized to a less toxic form of arsenic through a process of methylation.[3] Organoarsenic compounds arise via biomethylation of inorganic arsenic compounds,[4] via processes mediated by enzymes related to vitamin B12.[5] For example the mold Scopulariopsis brevicaulis produce significant amounts of trimethylarsine if inorganic arsenic is present.[6] The organic compound arsenobetaine, a betaine, is found in some marine foods such as fish and algae, and also in mushrooms in larger concentrations. The average person's intake is about 10-50 µg/day. Values about 1000 µg are not unusual following consumption of fish or mushrooms. But there is little danger in eating fish because this arsenic compound is nearly non-toxic.[7] Arsenobetaine was first identified in the Western rock lobster [8][9]
Carbohydrates bound to arsenic, collectively known as arsenosugars, are found especially in seaweeds. Arsenic containing lipids are also known.[10] Although arsenic and its compounds are toxic for humans, one of the first synthetic antibiotics was Salvarsan (the use of which has long been discontinued).
The only polyarsenic compound isolated from a natural source is arsenicin A.[11]
Organoarsenic compounds may pose significant health hazards, depending extremely on their speciation (LD50 ranging from 5–6 mg /kg bw (very toxic) to 12000–15000 mg / kg bw (practically non-toxic).[citation needed]
Representative compounds
Some illustrative organoarsenic compound are listed in the table below:
Organoarsenic R Molar mass CAS number Properties 10,10'-oxybis-10H-Phenoxarsine 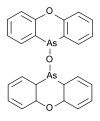
502.2318 58-36-6 Triphenylarsine Phenyl 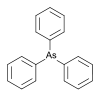
306.23 603-32-7 Melting point 58-61 °C Phenyldichloroarsine phenyl, chlorine 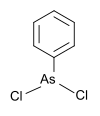
222.93 696-28-6 Roxarsone 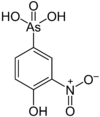
263.04 121-19-7 Arsenobetaine 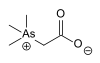
64436-13-1 Representative organoarsenic compounds [12] See also
- Arsenic biochemistry
- Arsenic poisoning
- Arsenic toxicity
- Category:Arsenic compounds
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Sabina C. Grund, Kunibert Hanusch, Hans Uwe Wolf "Arsenic and Arsenic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, VCH-Wiley, 2008, Weinheim.
- ^ a b Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-29390-6
- ^ Reimer, K.J.; Koch,I.;Cullen, W.R. (2010). "Organoarsenicals. Distribution and transformation in the environment". Metal ions in life sciences (Cambridge: RSC publishing) 7: 165–229. ISBN 9781847551771.
- ^ Dopp, E.; Kligerman, A.D.;Diaz-Bone, R.A. (2010). "Organoarsenicals. Uptake, metabolism and toxicity". Metal ions in life sciences (Cambridge: RSC publishing) 7: 231–265. doi:10.1039/BK9781847551771-00231. ISBN 9781847551771. PMID 20877809.
- ^ Toshikazu Kaise, Mitsuo Ogura, Takao Nozaki, Kazuhisa Saitoh, Teruaki Sakurai, Chiyo Matsubara, Chuichi Watanabe, Ken'ichi Hanaoka (1998). "Biomethylation of Arsenic in an Arsenic-rich Freshwater Environment". Applied Organometallic Chemistry 11: 297–304. doi:10.1002/(SICI)1099-0739(199704)11:4<297::AID-AOC584>3.0.CO;2-0.
- ^ Bentley, Ronald; Chasteen, Thomas G. (2002). "Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth". Microbiology and Molecular Biology Reviews 66 (2): 250–271. doi:10.1128/MMBR.66.2.250-271.2002. PMC 120786. PMID 12040126. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=120786.
- ^ Cullen, William R.; Reimer, Kenneth J. (1989;). "Arsenic speciation in the environment". Chemical Reviews 89 (4): 713–764. doi:10.1021/cr00094a002.
- ^ Francesconi, Kevin A.; John S. Edmonds Croatian Chemica Acta (1998). Arsenic Species in Marine Samples. 71. pp. 343–359. http://public.carnet.hr/ccacaa/CCA-PDF/cca1998/v71-n2/CCA_71_1998_343_359_FRANCES.pdf.
- ^ John S. Edmonds, Kevin A. Francesconi, Jack R. Cannon, Colin L. Raston, Brian W. Skelton and Allan H. White (1977). "Isolation, crystal structure and synthesis of arsenobetaine, the arsenical constituent of the western rock lobster panulirus longipes cygnus George". Tetrahedron Letters 18 (18): 1543–1546. doi:10.1016/S0040-4039(01)93098-9.
- ^ Alice Rumpler, John S. Edmonds, Mariko Katsu, Kenneth B. Jensen, Walter Goessler, Georg Raber, Helga Gunnlaugsdottir, Kevin A. Francesconi (2008). "Arsenic-Containing Long-Chain Fatty Acids in Cod-Liver Oil: A Result of Biosynthetic Infidelity?". Angew. Chem. Int. Ed. 47: 2665–2667. doi:10.1002/anie.200705405. PMID 18306198.
- ^ Mancini, Ines; Guella, Graziano; Frostin, Maryvonne; Hnawia, Edouard; Laurent, Dominique; Debitus, Cecile; Pietra, Francesco (2006). "On the First Polyarsenic Organic Compound from Nature: Arsenicin a from the New Caledonian Marine SpongeEchinochalina bargibanti". Chemistry - A European Journal 12 (35): 8989–94. doi:10.1002/chem.200600783. PMID 17039560.
- ^ www.sigmaaldrich.com
Categories:- Organoarsenic compounds
Wikimedia Foundation. 2010.
