- Maurotoxin
-
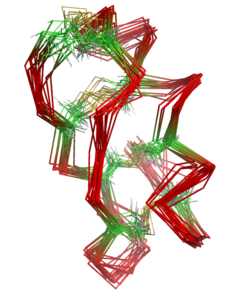
 The protein NMR structure of maurotoxin, illustrating the fluctuations in the protein's native state in solution. The protein backbone is shown in red, the alpha carbons of the eight cysteine residues in green, and the disulfide bridges in yellow. Compare the disulfide bond connectivity to HsTx1 below.
The protein NMR structure of maurotoxin, illustrating the fluctuations in the protein's native state in solution. The protein backbone is shown in red, the alpha carbons of the eight cysteine residues in green, and the disulfide bridges in yellow. Compare the disulfide bond connectivity to HsTx1 below.
Maurotoxin (abbreviated MTX) is a peptide toxin from the venom of the Tunisian chactoid scorpion Scorpio maurus palmatus, from which it was first isolated and from which the chemical gets its name. It acts by blocking several types of voltage-gated potassium channel.
Contents
Chemistry
Maurotoxin is a peptide of 34 amino acids cross-linked by four disulfide bridges, with an atypical pattern of organization compared with other scorpion toxins; this unusual pairing of cysteine residues may be mediated by the presence of adjacent prolines. The peptide contains an alpha helix linked by two disulfide bridges to a two-stranded antiparallel beta sheet.
Target
Scorpion toxins constitute the largest group of potassium (K+) channel blockers and are useful pharmacological probes to investigate ion channels and their functions.
Maurotoxin (MTX) blocks various K+ -channels:
- Apamin-sensitive small conductance Ca2+ - activated K+ channels (SK)
- Intermediate conductance Ca2+ - activated K+ channels (IK)
- Several types of voltage-gated potassium channels (Kv1.1, Kv1.2, Kv1.3 and shaker B)
The structural and pharmacological features of MTX suggest that MTX belongs to a new class of natural K+ channel blockers structurally intermediate between the Na+ (60–70 residues and four disulfide bridges) and K+ channel scorpion toxin families (less than 40 residues and three disulfide bridges).
The intermediate conductance Ca2+-activated K+ (IK) channel is present in peripheral tissues, including secretory epithelia and blood cells. An important physiological role of the IK channel is to help maintain large electrical gradients for the sustained transport of ions such as Ca2+ that controls T lymphocyte (T cell) proliferation. Thus IK blockers could be potential immunosuppressants for the treatment of autoimmune disorders (such as rheumatoid arthritis, inflammatory bowel disease and multiple sclerosis).
Mode of action
MTX occludes the pore region of various potassium channels (Kv1.2, IKCa1, Kv1.3) by establishing strong interactions between its lysine-23 residue and the glycine-tyrosine-glycine-aspartate (GYGD) motif of the channel. MTX thus blocks the channels by binding in the external vestibule of the pore to block the ion conduction pathway. Although Kv1.1, Kv1.2, and Kv1.3 have a very similar pore structure, they display different pharmacological sensitivity to MTX.
References
- Carlier, E., et al., Effect of maurotoxin, a four disulfide-bridged toxin from the chactoid scorpion Scorpio maurus, on Shaker K+ channels. J Pept Res, 2000. 55(6): p. 419-27.
- Castle, N.A., et al., Maurotoxin: a potent inhibitor of intermediate conductance Ca2+-activated potassium channels. Mol Pharmacol, 2003. 63(2): p. 409-18.
- Fu, W., et al., Brownian dynamics simulations of the recognition of the scorpion toxin maurotoxin with the voltage-gated potassium ion channels. Biophys J, 2002. 83(5): p. 2370-85.
- Jensen, B.S., et al., The Ca2+-activated K+ channel of intermediate conductance:a possible target for immune suppression. Expert Opin Ther Targets, 2002. 6(6): p. 623-36.
- Kharrat, R., et al., Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur J Biochem, 1996. 242(3): p. 491-8.
- M'Barek, S., et al., A maurotoxin with constrained standard disulfide bridging: innovative strategy of chemical synthesis, pharmacology, and docking on K+ channels. J Biol Chem, 2003. 278(33): p. 31095-104.
- Rochat, H., et al., Maurotoxin, a four disulfide bridges scorpion toxin acting on K+ channels. Toxicon, 1998. 36(11): p. 1609-11.
- Visan, V., et al., Mapping of maurotoxin binding sites on hKv1.2, hKv1.3, and hIKCa1 channels. Mol Pharmacol, 2004. 66(5): p. 1103-12.
Channel blocker: potassium channel blockers Antiarrhythmic III/delayed rectifier benzofuran (Amiodarone) • quaternary ammonium (Bretylium) • naphthalene (Bunaftine) • phenethylamine (Dofetilide) • sulfonamide (Ibutilide) • pyrimidinone (Nifekalant) • ethanolamine (Sotalol) • cyclopropane (Tedisamil) • E-4031Other/ungrouped/unknown aminopyridines (3,4-Diaminopyridine, 4-Aminopyridine) • indole (Linopirdine, Paxilline) • quaternary ammonium (Tetraethylammonium) • peptide (Maurotoxin, Charybdotoxin)Categories:- Neurotoxins
- Ion channel toxins
- Invertebrate toxins
Wikimedia Foundation. 2010.